Vaccines, drugs, and modified human cells that activate the immune system against cancer have improved outcomes and prolonged lives in some types of cancer in the past few years. For patients with glioblastoma, the most common primary brain tumor in adults, immunotherapy has shown some promise in clinical trials — but it can’t yet be called a major advance against this lethal malignancy.
That’s not surprising to specialists who treat glioblastoma, which resists most forms of cancer treatment. The tumors are made up of an array of different cell types, making them difficult to target, and they hide behind the body’s blood-brain barrier that excludes many drugs and cell types.
Nevertheless, researchers are testing an array of immunotherapy strategies such as vaccines, checkpoint inhibitor drugs, CAR T cells, and cancer cell-killing viruses in hopes of cracking the tumors’ defenses.
Learn More:
“Patients with brain tumors who benefit from immunotherapy drugs are a small percentage of the population that’s treated – maybe 5 to 10 percent with studies done so far,” says David Reardon, MD, clinical director of the Center for Neuro-Oncology at Dana-Farber. The fact that some patients do respond “is a validation that the immune system has the capability of helping in this disease,” he says, “but there is a long way to go to optimize that and make it a higher percentage of patients.”
Researchers believe drug combinations may work better, and the timing of treatment may result in different outcomes. Most of the immunotherapy trials carried out to date involved patients whose tumors had recurred following surgery and other treatments. The best time to do immunotherapy trials may be in the first-line setting where the immune system of the patient is more robust and surgery has removed as much of the tumor as possible, according to Patrick Wen, MD, director of the Center for Neuro-Oncology at Dana-Farber. Such early-stage trials are now recruiting patients.
As of 2017, no immunotherapy treatment for brain tumors has made it through late-stage clinical trials, and the Food and Drug Administration has not approved any immunotherapy drugs for glioblastoma. Opdivo, or nivolumab, one of the checkpoint inhibitor drugs that’s been successful in several types of cancer, failed when given by itself in a phase III trial for glioblastoma patients when compared against the targeted drug bevacizumab.
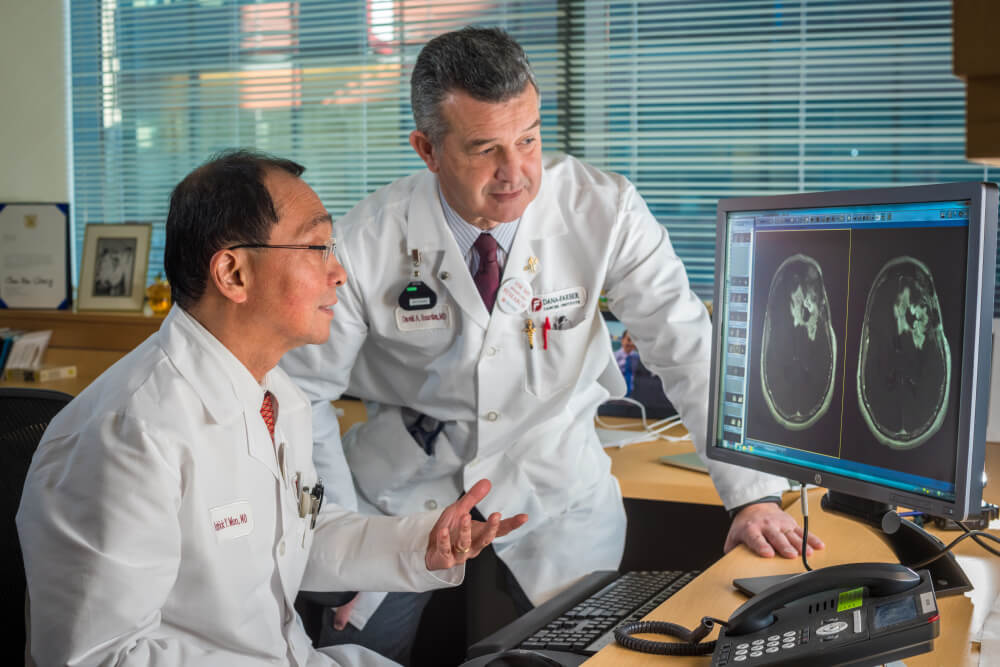
A different checkpoint inhibitor, durvalumab, is currently being evaluated in glioblastoma in a trial at Dana-Farber of which Reardon is the principal investigator.
One form of immunotherapy that’s shown some potential is personalized vaccines, which are tailored to genetic traits of an individual patient’s tumor and designed to train the immune system to seek out and attack the cancer.
In April 2017, researchers from Duke University reported intriguing results from a small study of a vaccine that targets a protein in glioblastoma cells expressed as a result of infection with the cytomegalovirus (CMV), which is very often found in the cells of glioblastoma tumors, but not in normal brain cells. In the study of 11 patients who received the vaccine, four patients survived for more than five years, which is highly unusual for glioblastoma.
At Dana-Farber, Reardon heads a trial of a personalized vaccine called NeoVax. In the trial, a specimen of a patient’s tumor undergoes DNA sequencing to identify mutations that create a distinctive pattern of “neoantigens” – molecules on the tumor cells’ surfaces that the custom-made vaccine can home in on. NeoVax is administered to patients following radiation and chemotherapy with temozolomide, and there are plans to add a PD-1 checkpoint inhibitor to the treatment. No results have been published yet, but Wen says he thinks the approaches “are really exciting.”
Still another vaccine, known as SurVaxM, is being tested in newly diagnosed glioblastoma patients in a phase II trial at Dana-Farber. SurVaxM, invented by researchers at Roswell Park Cancer Institute, stimulates the immune system to kill tumor cells that contain survivin, a protein that helps cancer cells resist conventional treatments.
While immunotherapy hasn’t yet proved superior to standard treatments for glioblastoma, scientists are hopeful that some of the many approaches they are testing will eventually lead to improvements in survival for brain tumor patients.
Learn more about immunotherapy and treatment for brain cancer from Dana-Farber.
