- The chromosomes in a some cancer cells are stranded in their own individual-sized nucleus, called a micronucleus.
- Researchers found that the envelope of the micronucleus is missing some critical materials, including key components of the nuclear-pore complex.
- The result is that pores are largely missing from the envelope, preventing entry of proteins needed to maintain the envelope and protect the chromosome from damage.
Desolate as a man stranded on a desert isle may be, he can at least be certain that, barring a tsunami, his refuge won’t crumble into the sea. The castaway chromosomes of cancer cells lack even that degree of assurance.
Unlike the chromosomes in normal cells, the chromosomes in some cancer cells aren’t always neatly packed inside the nucleus. A small number – often just one or two – are stranded in their own individual-sized nucleus, called a micronucleus. The envelope that surrounds the micronucleus is notoriously flimsy. When the envelope ruptures, as it often does, the chromosome inside tumbles into the cytoplasm – the jellylike material within the cell – where it’s broken into useless fragments. The release of genetic material can also draw an attack on the cells from the body’s immune system.
Scientists have puzzled over the fragility of the micronucleus envelope in contrast to the sturdier envelope around the cell’s central, primary nucleus. In a new study published in Nature, researchers at Dana-Farber supply an answer, not only demonstrating that the micronucleus envelope is built of inferior materials but also offering a scenario of how this occurs.
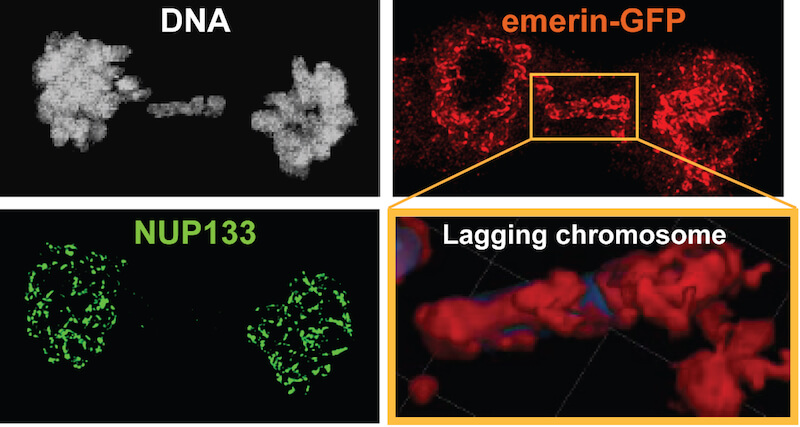
“One of the signature features of cancer cells is the erratic shape and structure of their nuclei,” says Dana-Farber’s David Pellman, MD, who led the study with Shiwei Liu and Mijung Kwon, PhD. “Micronuclei are an especially notable example of that irregularity.
“In previous research, we proposed that micronuclei are responsible for a condition known as chromothripsis, in which broad regions of a cell’s DNA are rearranged by a single catastrophic event. Chromothripsis occurs after the micronucleus envelope spontaneously shatters, fragmenting the chromosome inside. In this study, we wanted to explore why the envelope is so fragile and breaks apart so easily.”
Double layered protection
The tough, double-layered envelope of a cell’s primary nucleus consists of a variety of materials – proteins, lipid membranes, and structures called nuclear-pore complexes, which serve as gates for materials to enter and leave the nucleus.
Using lab-grown human cells, Pellman and his colleagues found that the envelope of the micronucleus is missing some critical materials, including key components of the nuclear-pore complex. The result is that pores are largely missing from the envelope, preventing entry of proteins needed to maintain the envelope and protect the chromosome from damage.
The researchers then turned their attention to the causes of shoddy envelope construction. “Some research had suggested that cells have a kind of quality-control system that verifies that chromosomes are in the right location after cell division,” Pellman relates. “We found, however, that the machinery for chromosome positioning is very poorly coordinated, and as a result, mistakes are irreversible. The process represents one of the major vulnerabilities in the cell life cycle, which can explain why chromothripsis is so common in cancer.”
With few genetic controls, the movement of chromosomes during cell division can easily go awry. As a cell divides, two sets of chromosomes – perfect copies of each other – bind to a structure called the spindle. The spindle, made up of threadlike protein structures called microtubules, stretches across the dividing cell and serves as a kind of conveyor belt, guiding one set of chromosomes to one end of the cell and the second set to the other end. Later, an envelope forms around the chromosomes to create a nucleus in each daughter cell.
Normally, each set of chromosomes travels along the spindle together. When the chromosomes reach the appointed location, they’re encased in a nucleus whose envelope is built by two “teams” of proteins, Pellman explains. Sometimes, however, a chromosome lags behind the others and is isolated in a micronucleus. Here, only a single team of proteins is available for envelope-making, resulting in a rather threadbare product.
Why is the second team AWOL? The likely answer will be familiar to anyone who has tried to squeeze through a fence. During the construction of the primary nucleus membrane, portions of the nucleus are in close contact with the spindle. The nucleus is assembled from materials arranged in sheets in the cell membrane. One team of proteins may be best suited to bring flexible pieces of membrane that can fit through the microtubule fence and reach the stragglers within the spindle. The other team might need large sheets of membrane that are blocked by the fence. The definitive answer awaits more work but could be as simple as physical blockage, researchers say.
“The absence of precise controls coordinating the movement of chromosomes with the assembly of the nucleus may explain why errors at this point of the cell cycle are prone to occur,” Pellman remarks, “and why catastrophic genomic rearrangements arise so frequently in cancer cells.”
