Patients with large B-cell lymphoma (LBCL) who did not respond to initial treatment or relapsed within a year may now receive the CAR T-cell product axicabtagene ciloleucel (Yescarta) as a second-line therapy, following approval by the U.S. Food and Drug Administration (FDA) based on results of a recent clinical trial.
Until now, the standard of care for patients with refractory or relapsed LBCL has been second-line chemotherapy, and for responding patients, subsequent high-dose chemotherapy and autologous stem cell transplantation. CAR T-cell therapies like axicabtagene ciloleucel, or “axi-cel” were reserved for third line use for patients who either did not respond sufficiently to second-line chemotherapy or who had relapsed following high dose chemotherapy and autologous stem cell transplantation.

However, in the Zuma 7 clinical trial, whose results were reported last December, Yescarta given as second-line therapy proved superior to the current standard of care, reducing the risk of disease progression, death, or the need for a new therapy by 60.2%. At two years, 40.5% of patients treated with Yescarta were still alive and didn’t require additional cancer treatment or experience cancer progression, versus 16.3% for the control arm.
“We observed a clear improvement with axi-cel, as compared with standard care, in event-free survival and the percentage of patients with a response,” said the authors of a report in the New England Journal of Medicine. Caron Jacobson, MD, MMSc, medical director of the Immune Effector Cell Therapy Program at Dana-Farber is third author of the report.
“This is a terrific advance for our patients who either don’t go into remission or relapse early after first-line chemotherapy,” says Jacobson. “These patients have a very low chance of benefiting from second-line chemotherapy but prior to the new approval they would not have been eligible for CAR T cells until they had failed to respond to chemotherapy. Now, we don’t need to administer ineffective therapy just to get them to their CAR T-cells in the end anyway,” Jacobson added.
The estimated 18-month event-free survival rate was 41.5% in the axicabtagene ciloleucel arm of the Zuma-7 trial and 17.0% in the standard therapy arm. The estimated median event-free survival was 8.3 months and 2.0 months, respectively. Differences in overall survival did not reach statistical significance with limited follow-up for this endpoint. Additionally, many patients who progressed on the standard of care arm went on to receive CAR T-cells in the non-trial setting under the existing FDA label for these therapies.
Challenges of large B-cell lymphoma
Large B-cell lymphoma is an aggressive, fast-growing form of non-Hodgkin lymphoma, affecting about seven of 100,000 people in the United States annually, mostly middle-aged or older adults. With appropriate treatment, about two-thirds of patients can be cured. However, patients who relapse or don’t respond to first-line therapy have a poor prognosis. Most patients in this situation are unable to receive high-dose chemotherapy and qualify for a bone-marrow or stem cell transplant.
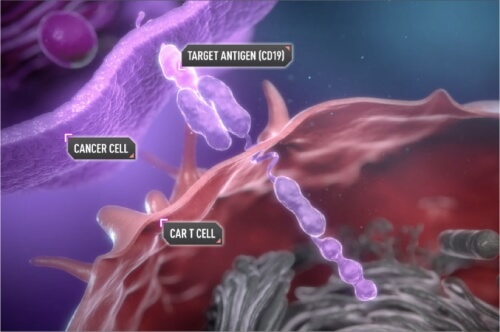
Yescarta, or axi-cel, manufactured by Kite Pharma, is made by removing the patient’s immune T-cells and sending them to the company’s laboratories, where they are engineered to equip them with a chimeric antigen receptor (CAR) that enables them to target the patient’s cancer. The CAR T cells are then re-infused into the patient’s circulation, where they seek out and attack cancer cells throughout the body.
Following the FDA’s approval on April 1 of Yescarta in the second-line setting, “referring physicians and patients can immediately begin accessing Yescarta CAR T-cell therapy for this new indication through Kite’s 112 authorized treatment centers across the U.S,” including Dana-Farber Brigham Cancer Center, the company said in a statement.
The safety of Yescarta treatment was described as manageable. However, the treatment caused high-grade adverse events in the vast majority of patients, though few had fatal effects. The incidence of cytokine release syndrome and neurologic events, which are known CAR T-cell side effects but not expected side effects of chemotherapy and autologous stem cell transplant, was higher in the axi-cel group.
