If the human immune system was a powerful racing car, you could say that scientists in the past few years have gained unprecedented control over how to make it accelerate, and what causes it to slow or stop. This knowledge has spawned new immunotherapy drugs that are delivering dramatic benefits to some patients with advanced cancers.
“Checkpoint blockers are transformational,” asserts Laurie H. Glimcher, MD, president and CEO of Dana-Farber and a prominent immunologist, referring to drugs that disable the brakes that cancer cells use to fend off an attack on them by immune system T cells.

“The idea that you can take someone who has stage IV metastatic cancer and halt the cancer – and manage it more like a chronic disease…it’s remarkable,” Glimcher says.
“However,” she adds, “it’s just the tip of the iceberg.” Beyond the impressive but limited successes of recent immunotherapy advances lies the potential to bring the strategy to more patients and more kinds of cancer.
The iceberg’s tip also represents current knowledge of the powerful immune system’s intricate and complex set of controls. Much of what will be needed to shape and steer the immune attack against cancers remains to be discovered.
“There’s so much we don’t understand,” Glimcher says. “Our task is to figure out the answer to at least two questions. First, why do only some patients with tumors that can respond to immunotherapy – like melanoma, lung, bladder and kidney – not respond to immunotherapy? Why is it only 20 or 30 or 40 percent? Why don’t all of them respond?
“And second, why do some cancers not respond at all, like pancreatic, prostate, ovarian, and breast cancer, glioblastoma, and colon cancer other than patients with Lynch syndrome?”
Despite those unanswered questions, the science behind immunotherapy is far more advanced than it was even a decade ago. For nearly 100 years, since the idea first emerged, efforts to harness the immune defenses as a cancer treatment met with many failures and limited success – even though the immune system, which evolved mainly to combat infectious viruses and bacteria, is capable of eliminating body cells that have become cancerous. Many strategies focused on stimulating the immune response with vaccines or removing T cells from a patient, “educating” them in the laboratory, and returning them to the body to seek out and destroy cancer cells. But except in a few instances, these measures didn’t spark an effective immune reaction.
It took what Glimcher calls an “Aha!” insight to jump-start the field of cancer immunotherapy.
That realization was that the best way to activate the immune system was not by stepping on the gas pedal – but by removing the brakes. Scientists learned that cancer cells evade the immune forces by activating molecular “checkpoints” that both conceal the identity of the cancer cells and switch off the immune response. These natural checkpoints are crucial to health – without them, people would be much more vulnerable to misguided attacks on normal tissue, as in autoimmune diseases like lupus. The role of one of those checkpoints on cancer cells, PDL-1, was identified by Dana-Farber’s Gordon Freeman, PhD, who in 2000 discovered that it partnered with another molecule on T cells, PD-1, to stave off attack by immune T cells. Another checkpoint, CTLA-4, also switches off the immune response.

“The T cells can get exhausted, and go into a state where the tumor is masked from the immune system, or the tumor secretes substances that create a highly immunosuppressive microenvironment” around the tumor, like a moat around a castle, says Glimcher.
The “moat,” a microenvironmental barrier that prevents killer T cells from invading the tumor, is composed of many kinds of suppressor cells including macrophages, dendritic cells, endothelial cells, and others. Glimcher’s own research has identified key molecular signaling pathways in the tumor microenvironment that are hijacked by cancer cells as protection; she and others are exploring strategies for “reprogramming” the environment to boost the immune response against tumors.
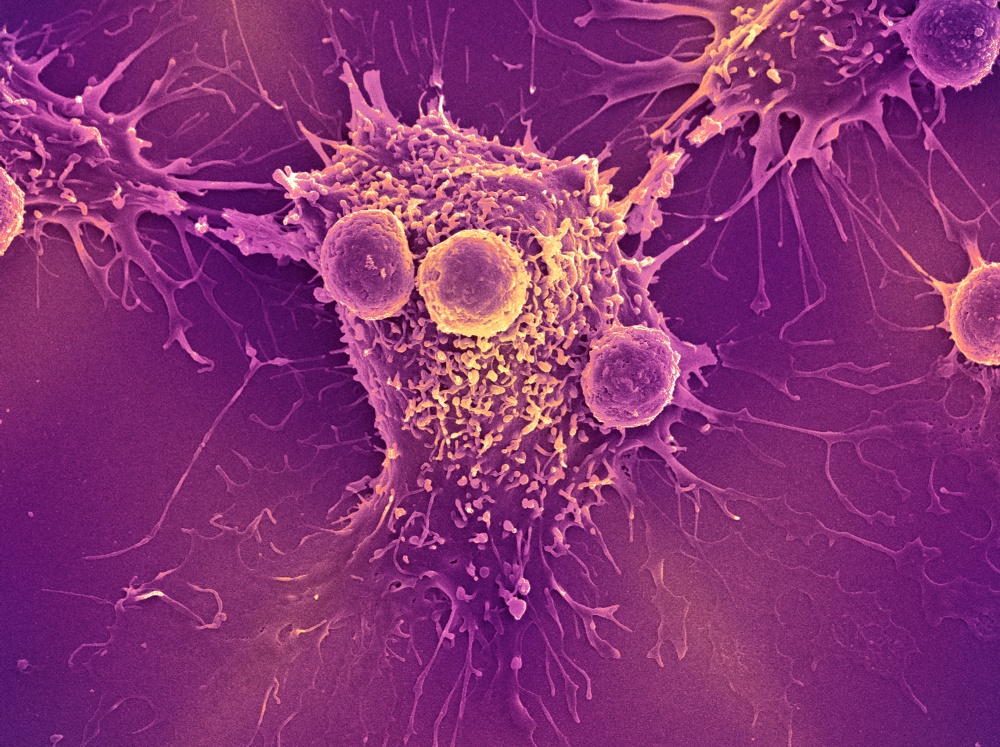
The discoveries of checkpoints that allow cancer to escape immune attack rapidly led to the development of “checkpoint blockade” antibody drugs that free T cells to attack and kill cancer cells. Dana-Farber’s F. Stephen Hodi, MD, director of the Center for Immuno-Oncology, led a groundbreaking clinical trial showing that ipilimumab (Yervoy), which blocks CTLA-4, could slow advanced melanoma in a significant number of patients and prolong their survival. Several other antibody drugs that block the PD-1/PD-L1 interaction have been approved, including pembrolizumab (Keytruda), nivolumab (Opdivo), and atezolizumab (Tecentriq). These drugs have found a place in treating non-small cell lung cancer, kidney cancer, bladder cancer, head and neck cancer, and Hodgkin lymphoma, and are being tested in other forms of cancer.
While many other checkpoint blockers are in company pipelines or clinical trials, researchers are exploiting the power of the immune system in other ways.
One approach that has gotten a lot of attention because of some early dramatic successes is CAR T cells. The patient’s T cells are removed and genetically modified in the laboratory to produce special receptors on their surface that recognize a specific protein on tumor cells. Then billions of the CAR T cells are infused into the patient to seek out and destroy the cancer. In some patients with very advanced blood cancers this strategy has had remarkable success, but it also can produce severe side effects that need to be closely managed.
Cancer vaccines continue to intrigue immunologists. Even though there are effective vaccines against the human papilloma virus (HPV), which causes cervical cancer, and some head and neck and anal cancers, only a minority of people at risk have undergone vaccination, Glimcher says. “It’s really a crime,” she says. “No women should die of cervical cancer.” She says she believes effective vaccines for non-viral cancers are possible, but the field is still in its infancy. Such vaccines would provoke the immune system to react against proteins displayed on the surface of cancer cells.
“Ultimately,” says Glimcher, “I think the answer is going to be combination therapy, just as it was for HIV/AIDS. The key to turning HIV from a lethal disease to a chronic disease was realizing you have to attack it with several drugs at the same time. It’s going to be trying to figure out which drugs work in which patients, precision immuno-oncology both for the tumor and the immune system.”
The potential of immuno-oncology is just beginning to be realized. Uncovering more of the iceberg will take both a much more detailed understanding of how the immune response is controlled and the tools or treatments to manipulate it for clinical benefit.
“I can’t think of a place that’s better equipped than Dana-Farber to take this on,” Glimcher reflects. “We have fantastic researchers who work closely with clinicians. And we can actually generate drugs here – we can take a basic discovery in the lab, do a proof of principle assay in animals, identify tool compounds, and then our chemists can turn that into a drug that could go into humans. Very few institutions have this capability.”
This article originally appeared in Dana-Farber’s 2017 issue of Paths of Progress. Learn more about immunotherapy advances at Dana-Farber.

When will more effort be put into treating small cell lung cancer?
Dear Shirley:
Thank you for your comment. Immunotherapy is an ongoing area of research in small cell lung cancer, which has unfortunately not yet seen as much successes non-small cell. You can read an article on recent trials on the American Society of Clinical Trials site: http://www.ascopost.com/issues/october-10-2016/small-cell-lung-cancer-and-immunotherapy-a-change-is-coming-just-not-front-line-yet/
You may also view clinical trials for small cell lung cancer on the NIH website: https://www.cancer.gov/about-cancer/treatment/clinical-trials/search/r?t=C4917%7Csmall_cell_lung_cancer&z=&a=
We hope this is helpful and wish you the best.
-DFCI