Many cancers grow rapidly and uncontrollably because their cells overrun the molecular brakes that normally permit cells to divide only when they are needed to replace old ones. These brakes are regulated by a group of enzymes known as cyclin-dependent kinases (CDKs). Alterations causing overactivity of two of these enzymes, CDK4 and CDK6, are found in a variety of cancers.
Dana-Farber’s Matthew Meyerson, MD, PhD, Peter Sicinski, MD, PhD, the late David Livingston, MD, and colleagues helped identify the potential of CDK4/6 proteins as a target to block cancer cell division. Today, several CDK4/6 inhibitors are approved cancer medicines.
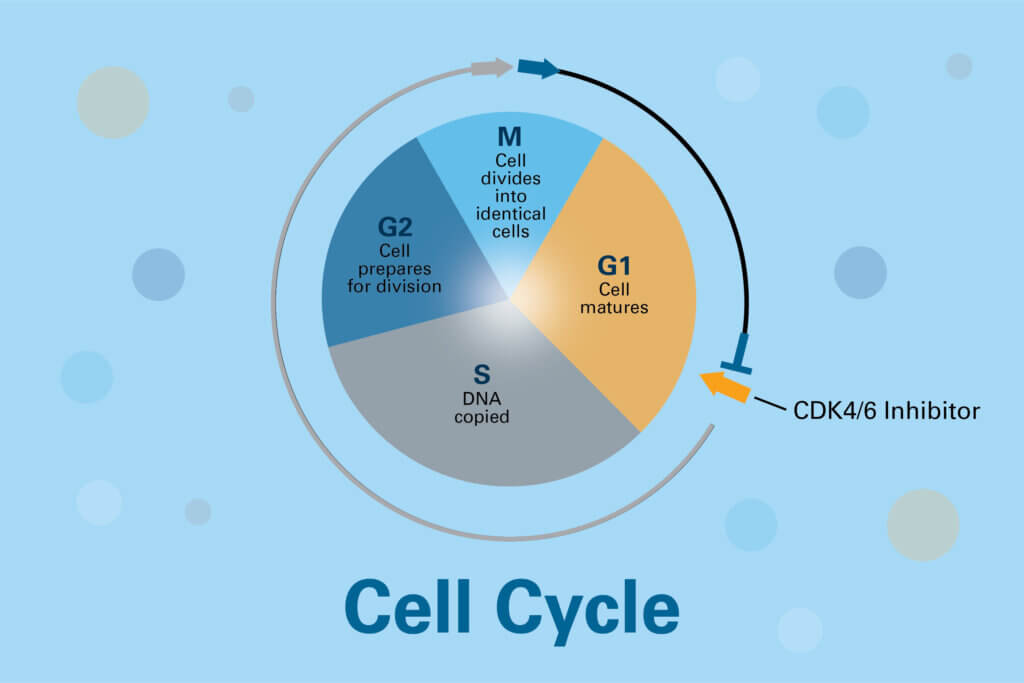
How do CDK4/6 inhibitors work?
The drugs work by selectively turning off the overactive CDK4 and CDK6 enzymes, according to Geoffrey Shapiro, MD, PhD, senior vice president of Developmental Therapeutics in Dana-Farber’s Center for Cancer Therapeutic Innovation. By reducing the activity of CDKs 4 and 6, these inhibitor drugs restore the growth-suppressive properties of the retinoblastoma (Rb) protein, which is a control point for cell division.
As a result, the cancer cells’ division cycle is halted, preventing them from proliferating. In some cases, the cancer cells not only stop dividing, but enter a state in which they lose all power to cycle and grow, after which tumors are able to shrink.
Research by Dana-Farber scientists has also revealed that CDK4/6 inhibitors fight tumors not only by blocking cell division, but also by unleashing the immune system to attack the cancer cells. Specifically, exposure to a CDK4/6 inhibitor can stimulate the production of substances that enhance the presentation of tumor antigens to the immune system’s cytotoxic T cells, allowing them to recognize the cancer cells as foreign and swarm to the attack.
The scientists also found that exposure to a CDK4/6 inhibitor reduced the population of another subset of T cells in the tumor microenvironment, known as immune-suppressing T regulatory cells, thereby helping to free the immune response against the cancer. The research further suggests that combining CDK4/6 inhibitor drugs with immune checkpoint-blocking antibodies may improve the effectiveness of immunotherapy.
What cancers can be treated with CDK4/6 inhibitors?
CDK4/6 inhibitors are approved for the treatment of breast cancer:
- The CDK 4/6 inhibitors palbociclib, ribociclib, and abemaciclib are approved for the treatment of hormone receptor-positive HER2-negative (HR+/HER2-) metastatic breast cancer.
- Abemaciclib has been approved for early-stage node positive HR+/HER2- breast cancer.
- Ribociclib has shown promising efficacy for early-stage HR+/HER2- breast cancer as well.
Clinical trials led by Dana-Farber’s Erica L. Mayer, MD, MPH, and Harold Burstein, MD, PhD, have explored the use of these CDK4/6 inhibitors. Combined with hormone-blocking drugs, the approved CDK4/6 inhibitors have substantially improved progression-free survival and response rates in patients.
CDK4/6 inhibitors have also demonstrated activity in other forms of cancer, including liposarcoma, non-small cell lung cancer, mantle cell lymphoma, melanoma, and glioblastoma.
Because CDK4/6 inhibitors appear to sensitize tumors to immunotherapy, combinations of CDK4/6 inhibitors with immunotherapy are under investigation in clinical trials. For instance, Mayer has led the PACE trial to explore the combination of palbociclib, endocrine therapy, and immunotherapy in patients with metastatic HR+/HER2- breast cancer, with results showing evidence of activity from the triplet therapy.
What is next?
Investigators are exploring combinations of CDK4/6 inhibitors with a variety of agents beyond immunotherapy agents to improve their effectiveness and to prevent the cancer from developing resistance to the drugs.
For instance, Ada Waks, MD, is leading a trial of a CDK4/6 inhibitor combined with a RAF/MEK inhibitor based on laboratory research at Dana-Farber suggesting that these inhibitors might prevent some patients with HR+ metastatic breast cancer from relapsing.
Rachel A. Freedman, MD, MPH, is also leading a clinical trial investigating the CDK4/6 inhibitor abemaciclib in combination with endocrine therapy in patients over age 70 with HR+ metastatic breast cancer. The goal of this study is to identify strategies to improve tolerability CDK4/6 inhibitors for older patients,
Several next generation CDK inhibitors are in early-stage clinical trials for HR+/HER2- breast cancer and other solid tumors.
Learn more about the clinical trials for breast cancer at Dana-Farber.
Learn more about Dana-Farber Brigham Cancer Center’s approach to breast cancer treatment.
About the Medical Reviewer

Dr. Mayer received her medical degree from Harvard Medical School, completed her residency in Internal Medicine at Brigham and Women's Hospital, and her fellowship in Medical Oncology at the Dana-Farber Cancer Institute. She also obtained a Masters in Public Health from the Harvard School of Public Health. She joined the staff of Dana-Farber and Brigham and Women's Hospital in 2006, where she serves as Director of Breast Cancer Clinical Research. She is a breast cancer medical oncologist and clinical investigator leading research studies focused on novel therapies for the treatment of breast cancer.
