The U.S. Food and Drug Administration has approved the CAR T-cell therapy called tisagenlecleucel (Kymriah™) for certain lymphoma patients. The treatment is for patients with relapsed or refractory large B-cell lymphoma including diffuse large B-cell lymphoma (DLBCL), high grade B-cell lymphoma and DLBCL arising from follicular lymphoma.
Kymriah is a highly specialized and personalized CAR T-cell therapy that uses specially altered T cells — part of the body’s immune system — to fight cancer. A sample of the patient’s T cells are collected from the blood and then modified to produce a special structure called chimeric antigen receptors (CARs) on their surface. When these CAR T cells are reinfused into the patient, the new receptors enable them to latch onto a specific antigen on the patient’s tumor cells and kill them.
“We’re making very important progress and I’m excited and encouraged to be able to provide another option for patients with no other effective treatments,” says Caron A. Jacobson, MD, Medical Director of the Immune Effector Cell Therapy program at Dana-Farber/Brigham and Women’s Cancer Center (DF/BWCC). DF/BWCC is a certified treatment center for the just approved CAR T-cell therapy.
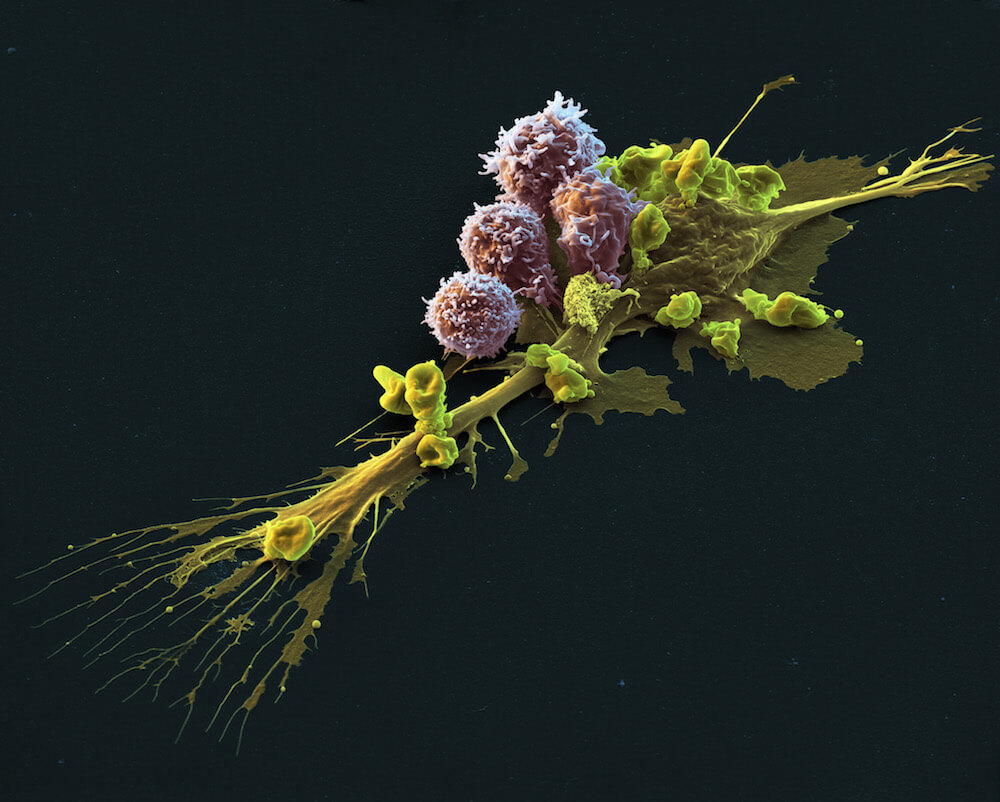
In clinical trials in patients with treatment-resistant or relapsed diffuse large B-cell lymphoma, 53 percent of patients responded to Kymriah, with 40 percent achieving a complete response – meaning no sign of cancer.
Kymriah was previously approved for patients with B-cell acute lymphoblastic leukemia (ALL) who are up to 25 years old. Last year, the FDA also approved another CAR T-cell therapy, Yescarta, for aggressive refractory adult non-Hodgkin lymphoma.
DF/BWCC and Dana-Farber/Boston Children’s Cancer and Blood Disorders Center are certified treatment centers for Kymriah for ALL. DF/BWCC is also a certified treatment center for Yescarta for lymphoma.
“Offering both Yescarta and Kymriah gives us flexibility and options in treating patients with these very challenging lymphomas,” said Jacobson.
Clinical trials of CAR T-cell therapy for blood cancers such as other types of lymphoma, multiple myeloma, and leukemia are underway at DF/BWCC, and include trials of CAR T-cell therapy earlier in treatment, and in combination with other immunotherapies.
Learn more about CAR T-cell therapy and lymphoma from Dana-Farber Cancer Institute.
