Key Takeaways:
- For years, scientists have explored the role of the protein BAX, which plays a significant role in cell death.
- Scientists have now identified a small molecule that interacts with BAX in a way that inhibits BAX’s activity.
- Researchers now have a starting point for the development of the first drugs for inhibiting BAX by a covalent mechanism.
Its nickname “the executioner protein” might give one the idea that BAX has a sinister role in cell life. But its killing has more to do with sacrifice than malice.
When a human cell is suffering from a lack of oxygen, a toxin, a genetic mutation, or other source of stress, it may kill itself for the good of the body through an elaborately choregraphed process called apoptosis. The death warrant passes through a protein chain of command that ultimately leads to BAX and its sibling-protein BAK. The two proteins then undertake a series of steps that result in the cell’s demise.
Under normal conditions, BAX is the most unassuming of proteins, lolling peacefully in the cell cytoplasm. But if blocked or overactive, it can cause a host of problems. In cancer, for example, BAX is often held captive by other proteins, enabling cancer cells to live and divide without being pestered by urges to die. In cases of stroke or neurodegenerative diseases, on the other hand, too much BAX can cause premature or unwanted cell death.
Its role in diseases as divergent as these as made BAX a prime object of scientific interest and a promising target for drug therapy. But BAX has proven defiantly difficult to study. It’s hard to make in a laboratory and even when it is synthesized successfully, it’s liable to twist itself into another shape without notice. (That changeability is actually critical to its role within the cell.)
In a new study, scientists at Dana-Farber and the University of California at San Francisco have identified a small molecule that interacts with BAX in a way that alters BAX’s activity. The molecule, dubbed Covalent BAX Inhibitor 1, or CBI1, is the first example of a covalent modulator of BAX – an agent that influences BAX by forming a chemical bond made of shared pairs of electrons. And, researchers say, it offers a blueprint for future drugs that can inhibit BAX to protect cells from unwanted death.
“BAX has been a focus of my lab for nearly 20 years,” says Loren Walensky, MD, PhD, senior author of the new paper, who began studying the protein as a postdoctoral fellow in the early 2000s. “Its enormous potential as a therapeutic target — if you activate it, you can cause malignant cells to die; if you shut it down, you can prevent healthy cells from dying — led us to explore its mechanism of activation and de-activation. Our findings in this study make a compelling case for the development of small molecule drugs to suppress BAX in diseases of premature or unwanted cell death.”
Shape-shifter
The broad outlines of BAX’s activity in the cell — how it’s called into action, how it responds to that call, and what happens next — have been the subject of intense investigation for years. Ordinarily, BAX lounges in a latent state in the cytosol, the water-like portion of the cell’s interior. When the cell comes under stress, BAX changes shape, becoming a toxic oligomer — composed of multiple associated versions of itself — that heads for the mitochondria, oblong structures that provide power to cell. It binds to the mitochondrial outer membrane and begins punching holes in it, allowing proteins that trigger apoptosis to escape.
Walensky likens BAX to a grenade. “It sits in the cytoplasm, locked down, waiting to be activated. When the signal comes, BAX is unleashed and the cell dies.”
Walensky’s team has dissected every step of that pathway, searching out its intricacies. They reported a major advance in 2020 in the journal Cell. Over the years, they have reported advances in dozens of publications, from Nature, to Molecular Cell, to Cell Reports, to Nature Communications.
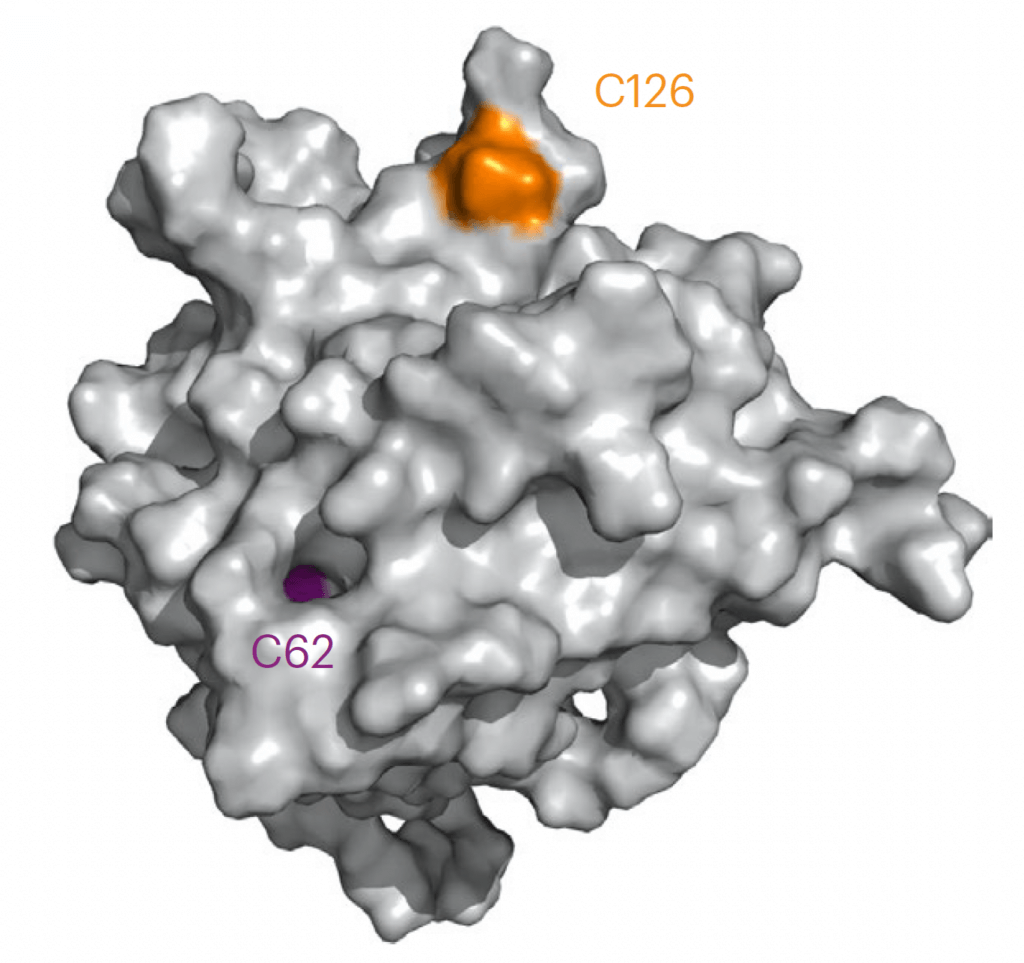
It turns out that BAX is activated when one of its cysteine amino acids, dubbed Cys 126, reacts covalently with a particular lipid molecule on the mitochondrial membrane. While the discovery was important for what it revealed about the triggering mechanism of BAX, it was even more significant for what it implied: Find a small molecule that reacts with BAX Cys 126, and it might be possible to control BAX itself — to upregulate or downregulate its activity.
Looking for a bridge
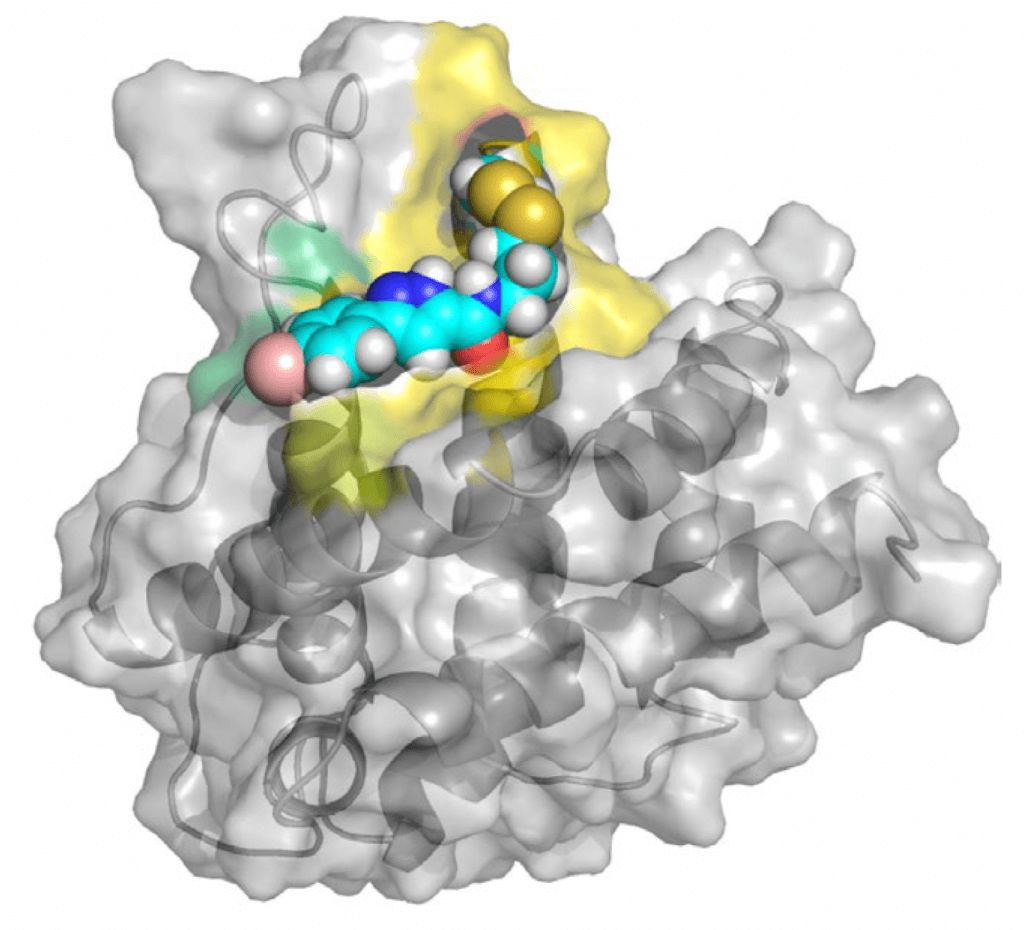
With the lab of James Wells, PhD, of the Helen Diller Family Comprehensive Cancer Center at the University of California at San Francisco, Walensky’s team screened hundreds of small molecules to see if any could form a disulfide “bridge” — a link between the molecules’ sulfur atoms and BAX Cys 126. They found one that established a particularly strong bridge — CBI1.
The researchers had good reason to believe that CBI1 could influence BAX’s activity, but whether it would dial that activity up or down was an open question. It turned out that unlike the mitochondrial lipid, which has a stimulatory effect, CBI1 suppresses BAX. These opposite effects occurred even though the lipid and CBI1 engage with the exact same cysteine on BAX.
“The first small molecule covalent mechanism shown to be capable of modulating BAX unexpectedly turns out to have an inhibitory effect,” Walensky states.
The researchers then showed that CBI1 achieves this effect through two distinct mechanisms:
1) When it reacts with Cys 126, CBI1 prevents BAX from changing shape.
Unable to shift from an idle configuration to an active one, BAX can’t become an oligomer that gouges holes in the mitochondria and triggers apoptosis.
2) CBI1 directly competes with the mitochondrial lipid to react with Cys 126. The result is that the lipid’s stimulatory effect on BAX is blocked.
With CBI1, researchers now have a starting point for the development of the first drugs for inhibiting BAX by a covalent mechanism. Such drugs would be especially valuable for treating diseases marked by a mass die-off of cells. They could also potentially be used to protect healthy cells from the damaging effects of cancer chemotherapy and radiation therapy, the researchers say.
