CAR T-cell therapy is a kind of cellular therapy, which uses a patient’s own immune system T cells to rally an attack on cancer. CAR T cells are made by removing T cells from the blood, modifying them in a lab to intensify the immune system’s natural response to cancer, and re-injecting them into the patient. CAR T therapy has produced exceptional results in patients with some types of blood cancer and is being tested against a variety of different cancer types.
CAR T-cell therapies are a form of immunotherapy that seeks to strengthen the immune system’s response to cancer. They differ from other immunotherapy agents known as immune checkpoint inhibitors, which aim to lower cancer cells’ defenses against an immune attack.
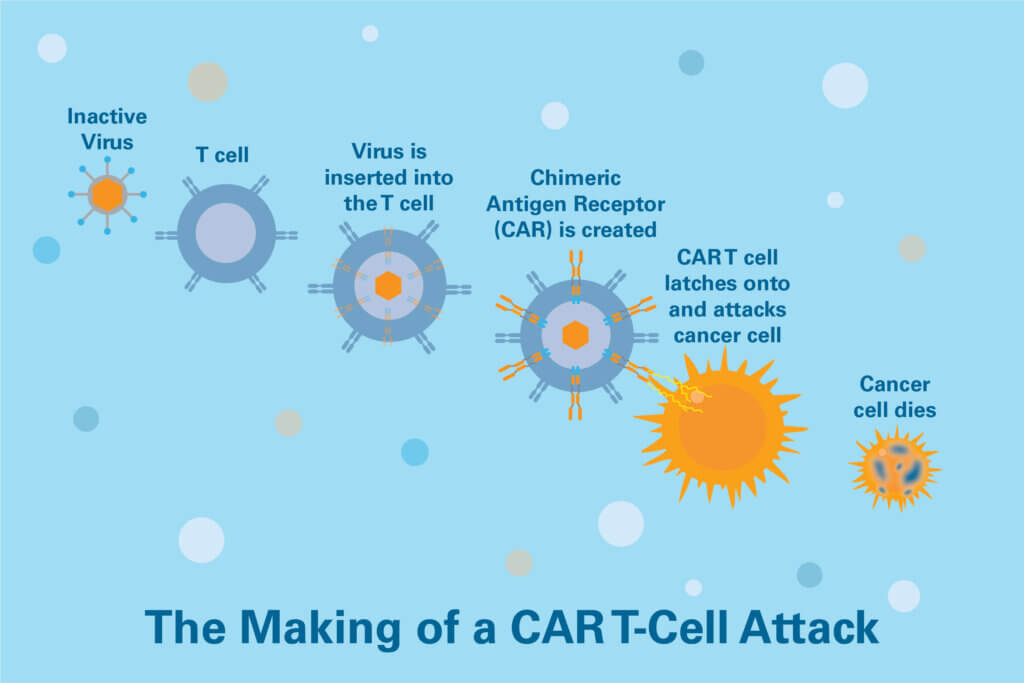
How does CAR T-cell therapy work?
CAR (for Chimeric Antigen Receptor) T-cell therapy uses specially engineered white blood cells called T cells to lead an assault on cancer. T cells’ role in the immune system is to hunt down and destroy abnormal cells, including cancer cells. For a variety of reasons, however, they don’t always recognize cancer cells, or don’t mount an all-out attack on them, potentially allowing tumors to take root and expand. Turning them into CAR T cells seeks to overcome those deficiencies.
To make CAR T cells, technicians collect a sample of a patient’s T cells from the blood and engineer them to sprout special structures called chimeric antigen receptors on their surface. When these CAR T cells are reinjected into the patient, the new receptors may help the T cells identify and attack cancer cells throughout the body.
Who is CAR T-cell therapy approved for?
CAR T-cell therapy has been approved by the U.S. Food and Drug Administration as standard therapy for:
- Some adult patients with aggressive non-Hodgkin lymphoma that has relapsed after prior treatments, or has not responded to other therapies (refractory).
- Some patients with relapsed or refractory mantle cell lymphoma.
- Some patients with relapsed/refractory follicular lymphoma.
- Patients with relapsed/refractory multiple myeloma.
- Some patients with relapsed/refractory chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL)
- Adult and pediatric patients with relapsed or refractory B-cell acute lymphoblastic leukemia (B-ALL)
Side effects of CAR T-cell therapy
CAR T-cell therapy has the potential to cause a range of side effects, which your care team can help manage.
CAR T-cell therapy can cause cytokine release syndrome (CRS), which can cause dangerously high fevers, extreme fatigue, difficulty breathing, and a sharp drop in blood pressure. Neurotoxicity, on the other hand, can result in side effects as mild as confusion, but can be much more severe — some patients have had difficulty with speaking and language, despite being alert, according to Caron Jacobson, MD, MMSc, medical director of the Immune Effector Cell program at Dana-Farber.
Neurotoxicity usually starts around six days after treatment, persists for three to 10 days, and then starts to improve. Other general side effects can include:
- Tremors
- Headaches
- Loss of balance
- Trouble speaking
- Seizures
- Sometimes, hallucinations
At Dana-Farber, care teams are highly trained in side effect management and can help patients deal with any issues that arise.
Outpatient CAR T-cell therapy
At Dana-Farber Brigham Cancer Center, some patients with lymphoma or multiple myeloma can receive CAR T-cell therapy on an outpatient basis. This is designed to be a more comfortable and convenient option for patients.
On Day 0, patients receive their CAR T cells. After being monitored for at least an hour, patients return to their “home base” — their home, hotel, or other living quarters — which must be within a 30-minute trip of Dana-Farber. Caregivers periodically monitor patients for possible side effects of the treatment: taking their blood pressure, oxygen level, and temperature, and answering questions on a question tool called an ‘ICE score’ that assess their cognitive ability. Patients must have a caregiver stay with them round-the-clock for up to a month and accompany them to hospital appointments.
Patients return to the Institute every day for laboratory tests and a clinical exam. These evaluations continue for a minimum of seven days. Patients experiencing side effects may be admitted to the hospital for closer monitoring. In addition to the in-person exam, patients have a daily afternoon phone check-in with a nurse to make sure they’re doing well and haven’t developed new side effects.
Allogeneic (or “off-the-shelf”) CAR T-cell therapy
CAR T-cell therapy can be dramatically effective, but as currently practiced, it has important limitations. For most currently available therapies, T cells must be removed from the patient and equipped with an engineered molecule in a specialized lab, then returned to the patient to fight a particular type of cancer. This typically takes 17 to 22 days — a period when “the patient has active cancer and might become too sick to get the CAR T cells while waiting,” says Jacobson.
“Also, the patients may have been heavily treated previously with other anti-cancer agents, which affect the health of their T cells that are the starting material for CAR manufacturing,” Jacobson continues. “And each time you manufacture autologous CAR T cells, it’s one dose, at a significant cost.”
To get around these drawbacks, researchers are in the early stages of developing a new generation of CAR T-cell therapies known as “off-the-shelf” treatments. Here, the immune cells are drawn from healthy donors and processed in labs, but then frozen and banked so they can be quickly administered to a cancer patient.
“The appeal of this allogenic therapy is that you can create a cell bank of engineered ‘master’ cells to deliver to the right patient within a short time frame,” says Sarah Nikiforow, MD, PhD, technical director of the Immune Effector Cell program.
There are, however, some concerns about the allogeneic approach that are being studied. The donor’s immune cells, while bearing CAR molecules, might also recognize the patient’s normal tissues as foreign, creating a risk of graft-versus-host disease, says Nikiforow. Another uncertainty is how long the donor CAR T cells will persist in the patient’s body, since they might be actively rejected by the patient’s immune system.
Dana-Farber investigators have been testing off-the-shelf CAR T cells in clinical trials for the past several years.

CART-T Cell Therapy.
Are there any trials underway for Ovarian Cancer?
Hi Mark:
Thanks for your comment. We do not currently have any clinical trials for CAR T-cell therapy in ovarian cancer, but you can search the National Cancer Institute for more clinical trial information: https://www.cancer.gov/about-cancer/treatment/clinical-trials/search
You may also view our current ovarian cancer clinical trials here: http://www.dana-farber.org/Research/Clinical-Trials/Clinical-Trials-by-Diagnosis.aspx?did=18
We wish you the best.
– DFCI