This post was originally published on Vector, Boston Children Hospital’s science and clinical innovation blog.
For more than 15 years, pediatric neuro-oncologist Mariella Filbin, MD, PhD, has been on a scientific crusade to understand DIPG (diffuse intrinsic pontine glioma). She hopes to one day be able to cure a disease that has historically been thought of as an incurable type of childhood brain cancer.
“While I was in medical school, I met a young girl who was diagnosed with DIPG,” Filbin recalls. “When I heard that there was no treatment available, I couldn’t believe that was the case. It really made a huge impression on me and since then, I’ve dedicated all my research to fighting DIPG.”
Her mission brought her to Boston Children’s Hospital for her medical residency program and later, to do postdoctoral research at the Dana-Farber/Boston Children’s Cancer and Blood Disorders Center. Now, she’s starting her own research laboratory focused on DIPG—which has also been called diffuse midline glioma (DMG) in recent years—and continuing to treat children with brain tumors at the Dana-Farber/Boston Children’s pediatric brain tumor treatment center. She’s also a scientist affiliated with the Broad Institute Cancer Program.
This year, Filbin has made new impact in the field by leveraging the newest single-cell genetic sequencing technologies to analyze exactly how DIPG develops in the first place. Her latest research, published in Science, entailed profiling more than 3,300 individual brain cells from biopsies of six different patients.
Using what’s known as a single-cell RNA sequencing approach to interrogate the makeup of DIPG/DMG tumors, Filbin was able to identify a particularly problematic type of brain cell that acts forever young, constantly dividing over and over again in a manner similar to stem cells.
Deciphering the Context of DIPG/DMG Tumors
Filbin—who was co-first author on the study—and her collaborators at the Broad and Massachusetts General Hospital were particularly interested in understanding the context of a gene mutation called H3K27M, which is typically found only in DIPG/DMG tumors. The mutation is particularly striking because it’s only found in brainstem tumors affecting young children. In contrast, it’s never found in adult brain tumors.
The team wondered: Could the context of the young, developing brain create a special opportunity for this mutation to trigger tumorigenesis? According to their analysis of thousands of individual brain cells comprising DIPG/DMG tumors, when the H3K27M mutation occurs in a particular brain stem cell known as an oligodendroglial precursor cell or OPC for short, it seems to keep them in a prolonged stem-cell like state.
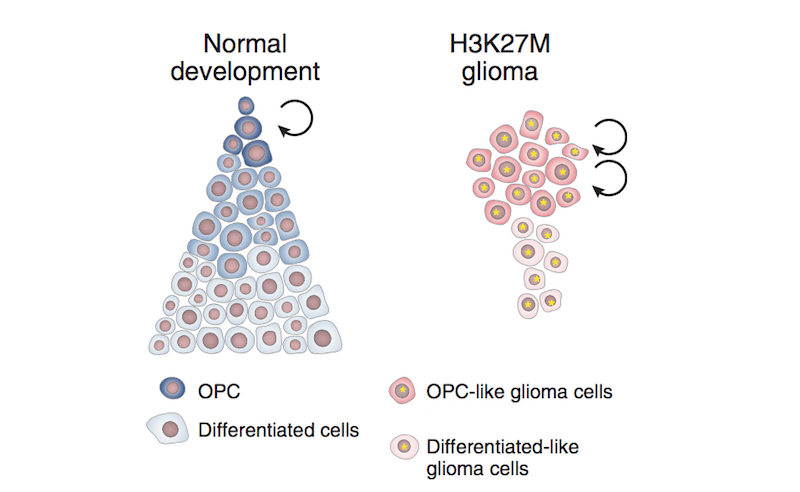
“In the case of DIPG/DMG, H3K27M mutations could force some cells to remain in a state of rapid proliferation, fueling tumor growth while stuck in this immature state,” Filbin says.
Unearthing a New Therapeutic Strategy for DIPG/DMG
The analysis also revealed a potential therapeutic strategy hidden within the context of the tumor cells. The team found that despite having the same H3K27M mutation, a select few cancer cells are still able to break free from their stem-cell-like state and differentiate into more mature cells.
The discovery was enabled by the team’s decision to use single-cell RNA-sequencing to sift through the many different cellular types found within a DIPG/DMG tumor. It provides a vastly-more precise view of each tumor’s complex landscapes than the historical sequencing approach used to interpret a tumor’s genomic profile, which involved pulverizing a tissue sample into a homogenous liquid and subsequently losing all context of the individual cells and their relationships to one another.
Although the team does not yet know why some cells are able to break out of the proliferation loop, the discovery brings hope to DIPG/DMG researchers. If they can determine the mechanism that enables some cells to differentiate despite the H3K27M mutation, Filbin’s team thinks that a future treatment strategy could employ a pharmacologic mechanism that would likewise prevent a child’s brainstem cells from getting stuck in an immature state.
“If we could find therapies that push all DIPG/DMG cancer cells out of this vicious stem-cell loop towards more normal behaving cells, that would be amazing,” Filbin says.
Learn more about DIPG from Dana-Farber/Boston Children’s Cancer and Blood Disorders Center.
