Key Takeaways:
- CAR T-cell therapy is a powerful addition to cancer immunotherapy and has offered new hope to patients with certain blood cancers.
- Scientists at Dana-Farber and elsewhere are looking to improve how CAR T-cell therapy is currently used, and to expand the use of CAR T cells to more blood cancers and solid tumors.
CAR T-cell therapy is a type of cellular therapy that uses a patient’s own immune system cells to rally an attack on cancer. This innovative treatment is approved for the treatment of some cancers, and researchers at Dana-Farber and beyond continue to study its potential.
The U.S. Food and Drug Administration has approved CAR T-cell therapies for some patients with non-Hodgkin lymphoma, including large-B-cell lymphoma, mantle cell lymphoma, and follicular lymphoma; B-cell acute lymphoblastic leukemia (B-ALL); multiple myeloma; and chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL).
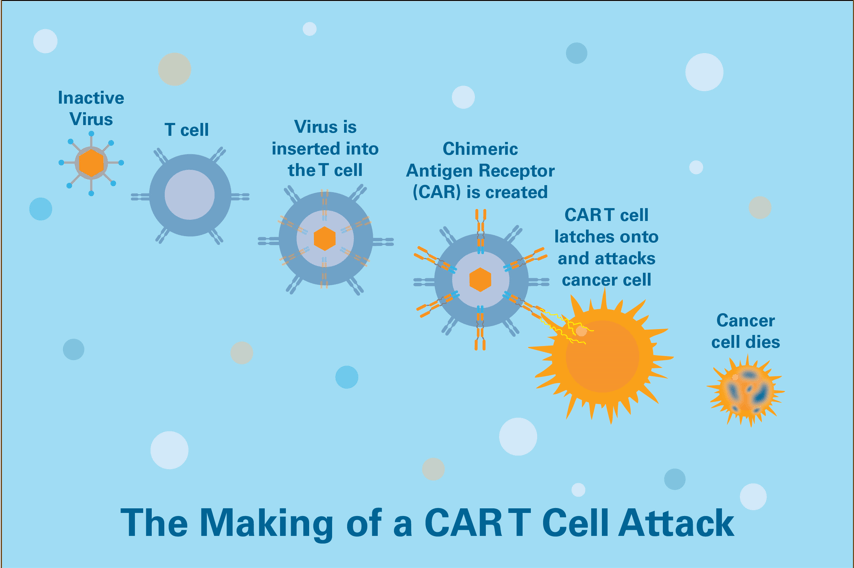
What are CAR T cells?
CAR T cells are made by removing some of the patient’s own immune cells and equipping them with an individualized laboratory-made receptor. When given back to the patient, these engineered cells act like a “living drug” — a one-time infusion of tailored T cells that home in on a specific protein on the patient’s cancer cells and mount an attack on the cancer.
While the treatments can have severe side effects, the availability of CAR T-cell treatments for some patients who otherwise had few options has been “totally transformative” and enables physicians to give a “message of hope,” says Caron Jacobson, MD, medical director of the Immune Effector Cell Therapy Program at Dana-Farber/Brigham and Women’s Cancer Center.
At present, the field is only in “the first leg of the CAR T journey,” as one assessment put it, with intense research underway to make the technology more widely applicable and safer.
Expanding CAR T-cell therapy to other cancers
Scientists believe there’s great promise for CAR T-cell therapy in blood cancers other than B-ALL, diffuse large B-cell lymphoma, and multiple myeloma and may be made effective against solid tumors as well. Several clinical studies are showing promise, Jacobson says.
In July 2020, CAR T-cell therapy (Tecartus) was approved for patients with mantle cell lymphoma following a clinical trial at Dana-Farber. Trial results showed that 87 percent of patients responded to the therapy, and 62 percent had a complete response. It has also been approved for some patients with acute lymphoblastic leukemia.
In March 2021, Yescarta was approved for patients with indolent relapsed/refractory follicular lymphoma. Trial results showed that 91 percent of patients responded to the CAR T-cell therapy, including an estimated 74 percent of patients in a continued remission at 18 months.
Abecma was also approved for patients with relapsed/refractory multiple myeloma in March 2021. The drug Carvykti has also been approved for patients with relapsed/refractory myeloma.
In March 2024, the FDA approved Breyanzi for patients with relapsed/refractory chronic lymphocytic leukemia or small lymphocytic leukemia. Trial results demonstrated that 45% of patients in the trial responded to the treatment and 20% had a complete response.
Clinical trials of CAR T-cell therapy are ongoing, exploring this therapy in combination with other therapies, giving CAR T earlier in the treatment cycle, and minimizing side effects.
CAR T cells that take aim at multiple targets
Initially, CAR T cells were engineered to recognize just one distinctive antigen on the surface of cancer cells, such as the CD19 antigen in B-cell ALL or the BCMA antigen in multiple myeloma. However, cancer cells can evolve to escape the immune attack by losing the primary target, making them invisible to the CAR T cells. Scientists are now devising CAR T cells that target multiple antigens in hopes of defeating this antigenic escape strategy.
Clinical trials and other research
Dana-Farber physician/scientists have recently opened several clinical trials of CAR T-cell therapies, with more trials due to open in the months ahead. As a group, they cover a wide range of areas – from evaluating existing CAR T-cell therapies in earlier lines for lymphoma and myeloma to therapies that target different proteins on tumor cells in the blood cancers where CAR T-cells are already approved as well as in solid tumors where there are no approved cell therapies to date.
Those blood cancer trials open or coming to Dana-Farber include:
- A randomized trial of CAR T-cell therapy as first-line treatment for patients with high-risk diffuse large B-cell lymphoma versus standard of care chemotherapy is open and enrolling. Participation in a randomized trial of BCMA CAR T cells versus autologous stem cell transplantation as a consolidation therapy in myeloma is being considered.
- Trials of CAR T-cell therapies for lymphoma that target cancer cell proteins other than CD19 or BCMA, which is the target for existing approved CAR T-cell therapies. In one such trial, involving CAR T cells that target the ROR1 protein, the CAR T cells will be manufactured at Dana-Farber’s Connell and O’Reilly Families Cell Manipulation Core Facility. The trials are open to patients whose lymphoma continues to advance after treatment with CD19-targeting CAR T-cell therapies. In another trial, a CAR against the myeloma protein GPRC5D developed in part by Dana-Farber’s Eric Smith, MD, PhD, is being investigated in a multicenter phase 2 study in relapsed myeloma.
- A trial of a novel CD19 CAR T-cell therapy using decentralized manufacturing (where CAR T cells would be manufactured on-site at each site participating in the trial) for patients with chronic lymphocytic leukemia and Richter’s transformation is being considered.
Dana-Farber researchers expect positive data to emerge from a now-closed trial of a CAR T-cell therapy called obe-cel for patients with acute lymphoblastic leukemia. They’re hopeful the data, when published, will lead to approval of the therapy for patients with this form of leukemia.
‘Off-the-shelf’ CAR T cell products
Research is underway to overcome a major limitation of current CAR T-cell therapy: using the patient’s own immune cells to create the modified T cells.
For one thing, the patient’s immune cells may not be the most potent, due to the cancer and its prior treatments, and for another, it takes two to four weeks to process the cells before they can be given back to the patient. As a result, scientists are working toward “off-the-shelf” CAR T cell products — made from the immune cells of a healthy donor, frozen and stored. These CAR T cells could be given almost immediately when a patient needed them, says Jacobson. One healthy donor’s cells could also potentially be used to make CAR T cells for as many as 100 patients.
Addressing side effects
Researchers are also working to understand, predict, prevent, and treat the toxic side effects that can be triggered after CAR T cells are infused into patients. Chief among them is cytokine release syndrome (CRS), a systemic inflammatory response that can be mild or severe and cause a number of complications such as fever, fatigue, low blood pressure, rapid heartbeat and pain.

Will t-cell therapy work for peritoneal carcinmotosis?
Hi Dana,
Thank you for reaching out. As of May 2018, CAR T-cell therapy has been approved by the U.S. Food and Drug Administration as standard therapy for some adult patients with aggressive non-Hodgkin lymphoma that has relapsed after prior treatments, or has not responded to other therapies (refractory), and for patients age 25 and under with relapsed or refractory B-cell acute lymphoblastic leukemia.
Cancers that arise from the peritoneal surface, called “peritoneal cancers,” are treated identically to ovarian cancer and fallopian tube cancer. You can find more information here: http://www.dana-farber.org/ovarian-cancer/about/
We are wishing you all the best,
DFCI