Many of the drugs that have become mainstays of cancer treatment are based on antibodies — artificial proteins that latch onto a molecular target called an antigen. When an antibody binds to an antigen on a cancer cell, it attacks the cell or triggers its destruction by immune system process.
A relatively new twist on this technology is bispecific antibodies, which are rapidly being tested and moving toward clinical use. Today, one bispecific antibody is approved by the U.S. Food and Drug Administration for certain patients with acute lymphoblastic leukemia (ALL), but they are showing promise in early trials for patients with other types of leukemia, non-Hodgkin lymphoma, and multiple myeloma.
How do bispecific antibodies work?
Bispecific antibodies can bind to two different targets on two different cells at the same time. This is different from most antibody drugs that are currently in wide use in immunotherapy that are limited to latching on to a single antigen on a single cell.
For example, some of these antibody drugs latch onto a protein called PD-L1, which is part of a system that cancer exploits to shut down the immune response. Antibodies that target PD-L1 remove the brakes so the immune system — mainly white blood cells called T cells — can attack the cancer.
Bispecific antibodies expand the power of antibody drugs by grabbing two molecules at once, which opens up a new avenue of therapeutic possibilities.
For example, one class of bispecific antibodies — termed bispecific T-cell engagers, or BiTEs — can latch onto a T cell and a tumor cell simultaneously. This close proximity enables the T cells to directly attack and kill the tumor cell.
Normally, T cells can only go on the attack after an antigen — a piece of the microbe or cancer cell — has been “presented” to it by another specialized cell called a dendritic cell. The bispecific antibody bypasses that step and brings the tumor cell and T cell together without the intermediate step.
“With bispecific antibodies, you’re bringing the T cell to the game,” says Daniel DeAngelo, MD, PhD, chief of the Division of Leukemia at Dana-Farber Cancer Institute. “These T-cell engagers bring the patient’s immune cells to target the relapsed cells. The advantages are that you can administer it whenever you want, and you can always turn it off.”
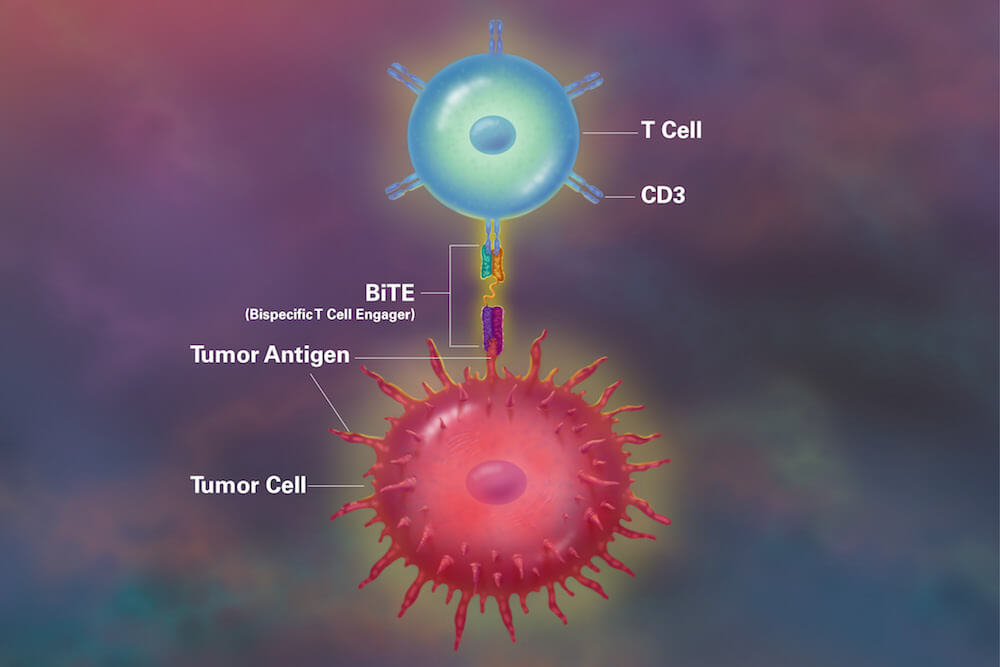
One such drug, blinatumomab, has been FDA-approved to treat adult and pediatric patients with a certain form of ALL. Blinatumomab has one segment that binds an antigen on B- lymphocytes and other to the CD3 antigen on T cells. It is indicated to treat patients with B-cell ALL in first or second complete remission or relapsed/refractory B-ALL.
What’s the latest in bispecific antibody research?
Bispecific antibody drugs are being developed and tested blood cancers such as non-Hodgkin lymphoma, acute myeloid leukemia (AML), and some solid tumors, such as small cell lung cancer and glioblastoma.
In late 2020 at the annual American Society of Hematology meeting, researchers said bispecific antibodies achieved impressively high response rates in patients with refractory B-cell non-Hodgkin lymphoma (B-cell NHL).
“This is an exciting time for bispecific antibodies in lymphoma,” says Jennifer Crombie, MD, a physician and clinical investigator in the Lymphoma Division at Dana-Farber. Dana-Farber researchers are currently testing bispecific antibody drugs for non-Hodgkin lymphoma in multiple clinical trials. There are currently two trials of bispecific antibodies, IgM-2323 and odronextamab, available for patients with relapsed/refractory B-cell NHL and others in development. There is also a study of the bispecific antibody mosunetuzumab in combination with chemotherapy for the initial treatment of patients with high-risk diffuse large B-cell lymphoma (DLBCL).
Bispecific antibodies could be an “off-the-shelf” form of immunotherapy if approved, unlike another immunotherapy strategy, autologous CAR T-cell therapy. The latter, which has been remarkably successful in treating some advanced blood cancers, involves a process in which the patient’s immune cells are removed and genetically altered in the laboratory, then returned to the body to seek out and attack cancer cells. This is a process that must be individualized for each patient; Bispecific antibodies are drugs that can be prescribed without having to be manufactured using the patient’s own immune cells.
Jacob Laubach, MD, clinical director for the Multiple Myeloma Program at Dana-Farber, has been involved in the development of Cevostamab, a novel bi-specific antibody targeting FcRH5/CD3 in patients with relapsed and refractory multiple myeloma. Data presented at the 2020 ASH meeting showed a high rate of response in patients who had been exposed to numerous prior lines of treatment.
“The level of activity of Cevostamab in patients with advanced disease was impressive and validates FcRH5 as a target in this disease,” Laubach says.
Trials evaluating other bi-specific antibodies in relapsed myeloma have likewise demonstrated a high level of anti-tumor activity.
“Bispecific antibodies represent a very exciting and promising new approach,” says Paul Richardson, MD, clinical program leader in the Jerome Lipper Multiple Myeloma Center at Dana-Farber. “But it’s important to note that it’s not one versus the other — we will need both CAR T cells and bispecific antibodies in multiple myeloma.”
