In the annals of patients who have benefited from Dana-Farber and Brigham and Women’s Hospital’s (BWH) genomic sequencing program Profile, few involve a turnabout as dramatic as one recently reported in Gynecologic Oncology.
Authored by nearly a dozen Dana-Farber and BWH faculty, the paper recounts the medical history of a 49-year-old Nebraska woman first diagnosed with metastatic endometrial cancer in 2015. Her treatment over the next three years was a combination of surgery and chemotherapy, relapse, re-treatment, and relapse before she arrived at Dana-Farber/Brigham and Women’s Cancer Center (DF/BWCC) for a recommendation on next steps.
At DF/BWCC, she underwent extensive surgery to remove metastatic growths from several locations in her abdomen that were causing considerable discomfort. Although no visible tumor remained after the surgery, pathology tests showed the cancer had invaded the patient’s stomach, pancreas, and other abdominal structures.
Tissue samples from the original tumor and one of the metastases collected during surgery were analyzed by Profile, which tests tumors for nearly 500 genomic abnormalities linked to cancer. Testing showed both the original and recurrent tumors to be “ultramutated,” or strewn with unusually high numbers of mutations and other irregularities — 231 in the original tumor, 305 in the recurrent tumor tissue. One such mutation, in a gene called POLE, drew researchers’ particular attention.
“Research has suggested that endometrial tumors with POLE alterations and high tumor mutation rate are susceptible to immunotherapy drugs known as checkpoint inhibitors,” says the paper’s first author, Jennifer Veneris, MD, PhD, of the Susan F. Smith Center for Women’s Cancers. “The presence of POLE mutation in the tumor suggested that our patient might be a good candidate for this therapy.”
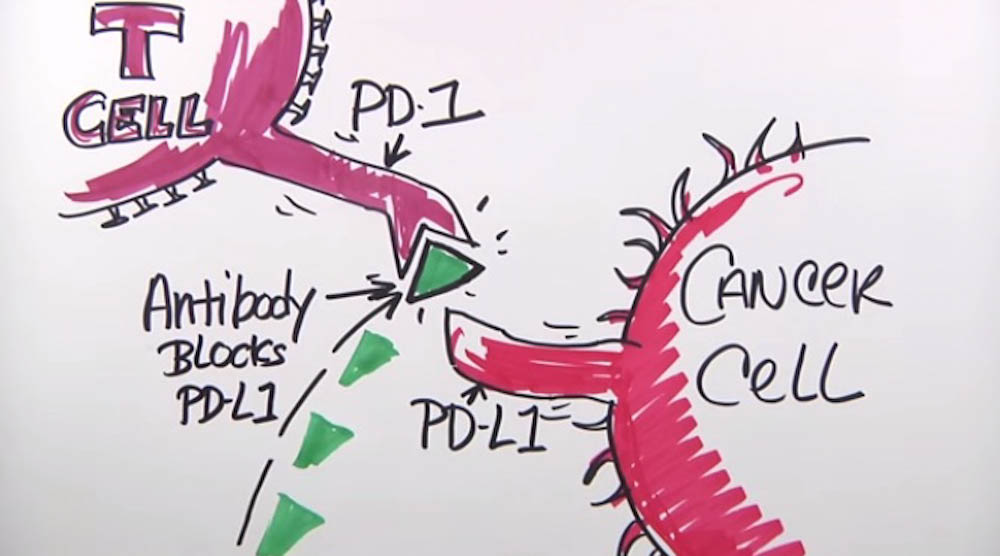
Within three months of the surgery at DF/BWCC, the cancer had recurred at multiple sites. On the basis of the Profile results, physicians treated the patient with the checkpoint inhibitor pembrolizumab. Within a short time, CT scans of her chest, abdomen, and pelvis showed a sharp diminution in all her lesions — some of them shrinking to less than a third of their original size. Today, after completing six cycles of pembrolizumab and remaining on the therapy, she continues to improve, Veneris says.
“In this case, standard histological or immunohistochemical testing would not have revealed a POLE mutation,” she remarks. “It’s the kind of marker that could only come to light with the type of genomic sequencing done in Profile.”
Co-senior authors of the study are Dana-Farber’s Susana Campos, MD, and Panagiotis Konstantinopoulos, MD, PhD. DF/BWCC co-authors are Elizabeth Lee, MD; Emily Goebel, MD; Marisa Nucci, MD; Neal Lindeman, MD; Neil Horowitz, MD; Larissa Lee, MD; Chandrajit Raut, MD; and Ursula Matulonis, MD.

Great results!!! Congratulations! My question is: Could this have been picked up earlier with genetic testing or did the mutation occur as a result of the previous chemotherapy or is that a question that is at this time unanswerable because all of her tissues were probably fixed in formalin, killing all the live cancer cells or weren’t saved at all…Thanks and great work!!