Cancer is often fueled by hormones, including the male sex hormone testosterone, which spur tumor growth in most forms of prostate cancer. Doctors can defuse this destructive relationship, typically with drugs (or sometimes surgery), but frequently, the tumors adapt or evolve, devising ways to incite cancer growth even in the absence of hormone-driven signals. These so-called castration-resistant tumors are especially challenging to treat and represent an important frontier in the discovery of new prostate cancer treatments.
“We’re interested in understanding the mechanisms that underlie the growth of these castration-resistant prostate cancers with the hope that it will lead us to clues about how to treat them better,” says Myles Brown, MD, Emil Frei III Professor of Medicine at Dana-Farber Cancer Institute.
Brown and his colleagues recently reported the results of an effort to dissect the genetic and molecular underpinnings of castration-resistant tumors. They focused their study on a truncated version of the androgen receptor, known as ARv7. Even in the absence of testosterone and other androgen hormones, Arv7 can signal tumor growth, and is often elevated in tumors from patients with castration-resistant prostate cancer. Their findings, which appeared in the March 18 issue of Cancer Cell, shed light on the biology of this aggressive cancer and point to some novel potential treatment strategies.
Brown led the study together with senior co-authors Anna Groner, a former postdoc in his lab, and Stephen Plymate, a professor at the University of Washington School of Medicine. The team began by cataloging all of the spots in the genome where ARv7 and its normal counterpart, the androgen receptor (AR), bind. (Both AR and ARv7 are classified as transcription factors, which bind to DNA and help turn genes on or off.) Once they defined the full set of AR and ARv2 binding sites (known as “cistromes”), Brown and his colleagues then combined the data with other types of large-scale analyses, including gene expression studies, to reveal which genes and groups of genes are regulated by AR and ARv7. With this side-by-side comparison of the two androgen receptors, some interesting trends emerged.
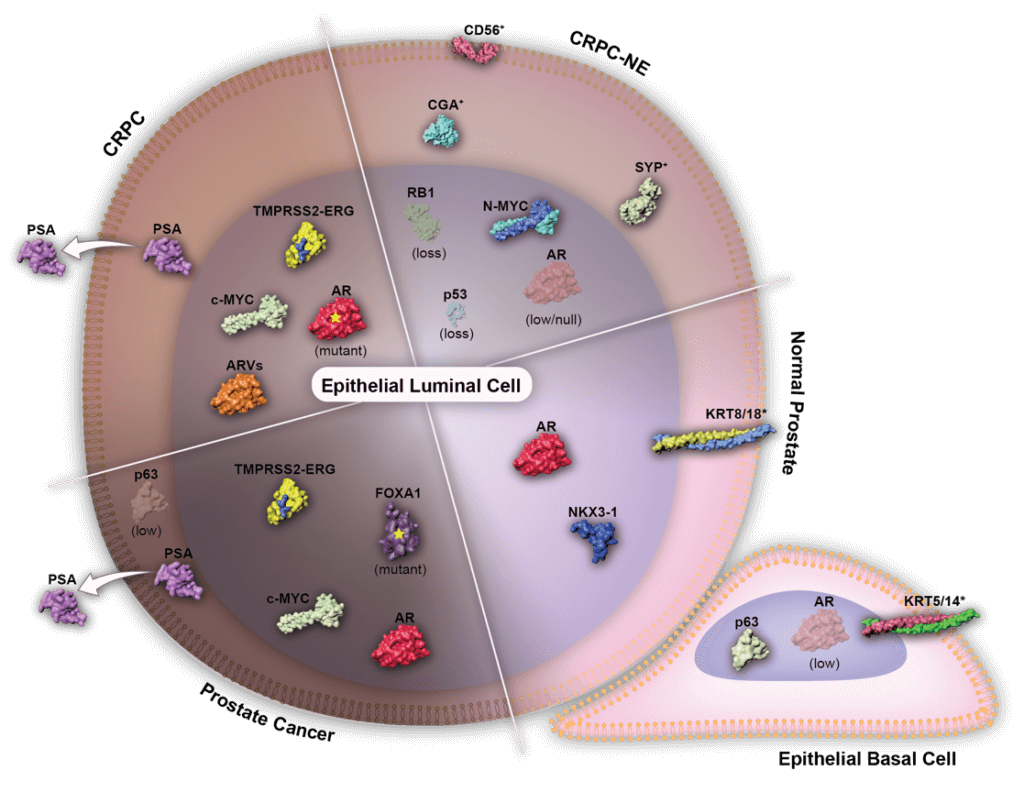
throughout disease progression.
“We found that most of the genes that were being regulated were actually controlled by the combination of ARv7 and the full-length AR working together,” said Brown.
But instead of turning genes on, the ARv7/AR combo turns genes — in particular, tumor suppressor genes — off.
Importantly, when Brown and his colleagues looked at tumors from patients with castration-resistant prostate cancer, they found that the activity levels of these ARv7/AR-controlled tumor suppressor genes appear to correlate with outcomes: patients tended to do better when the genes were on; when they were off, patients fared worse.
“Our work suggests that a better androgen receptor blocker — one that can block the activity of the full-length receptor together with v7 — could have therapeutic activity in men with castration-resistant prostate cancer,” said Brown.
Since some androgen-blocking therapies already exist and are in clinical use, the side effects of this treatment approach are known and there are some strategies to mitigate them. Moreover, ARv7 represents an active area of investigation not just in academia but also in the biopharmaceutical industry, which is also seeking ways to tackle resistant forms of hormone-driven cancers.
