Two years ago, with a new wife and large extended family on his mind, Jose Dos Anjos told caregivers he would do anything to help himself and others facing the aggressive, life-threatening blood cancer known as mantle cell lymphoma (MCL). Now, thanks in part to this willingness, he and fellow MCL patients have renewed hope for their futures.
On July 24, the U.S. Food and Drug Administration (FDA) approved the first CAR T-cell therapy for mantle cell lymphoma, representing a key advance for individuals with relapsed or treatment-resistant forms of the disease. Dana-Farber investigators helped conduct the decisive clinical trial for the therapy, and Dana-Farber/Brigham and Women’s Cancer Center (DF/BWCC) will be a certified treatment center for the therapy, known as KTE-X19. FDA approval means KTE-X19 can now be used as part of standard treatment for adult patients with MCL that has either relapsed or does not respond to other treatments.
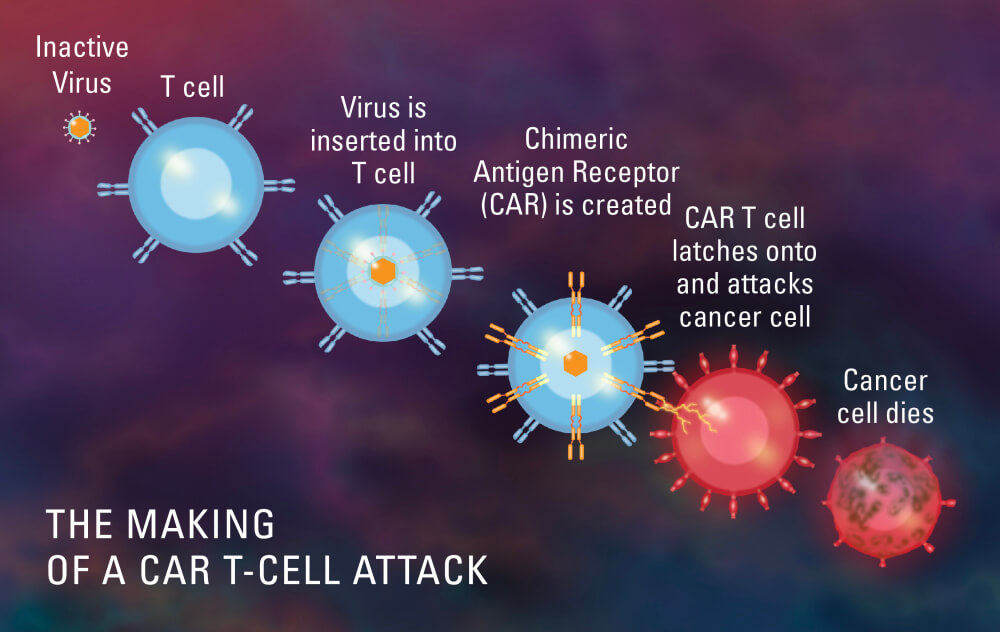
The ZUMA-2 clinical trial involved 74 adult patients — including Dos Anjos — whose mantle cell lymphoma had relapsed or become resistant to several prior lines of therapy. In the trial, 93% of participants responded to a single infusion of the agent, including 67% who achieved a complete response, or disappearance of all signs of their cancer.
“This is an incredibly exciting advancement in the treatment of mantle cell lymphoma, which is historically an incurable lymphoma with relatively short survival when chemotherapy stops working,” says Caron Jacobson, MD, medical director of the Immune Effector Cell Therapy Program at Dana-Farber and an investigator involved in the trial. “The responses seen in the ZUMA-2 trial in very high risk and heavily pretreated MCL patients are phenomenal, and although longer follow-up is needed, many persist beyond the one-year mark suggesting that this therapy has the potential to make a substantial impact on the natural history of this disease.”
Complete remission
When Dos Anjos, 57, found a lump under his armpit in November 2017, he did not initially get it checked “because it didn’t hurt.” Abdominal and back pain eventually led the Brockton, Mass. resident to his primary care physician, and tests resulting in his diagnosis.
Mantle cell lymphoma is a rare form of non-Hodgkin’s lymphoma, so-named because it originates in the “mantle zone” of the lymph node — a ring of cells surrounding an area where antibody-making B cells grow and mature. It is generally a disease seen in patients in their 60s and 70s and is treated with chemotherapy often in combination with other agents.

Dos Anjos had stage IV MCL, involving his bone marrow, spleen, and stomach. Referred to Dana-Farber for treatment, he went through two different courses of chemotherapy starting in January 2018. After an initial partial remission, his disease began progressing again — qualifying him for the trial. He received his KTE-X19 CAR T cells on November 13, 2018 at Brigham and Women’s Hospital, and was discharged home nine days later.
“Jose had an outcome that everyone hopes for when starting any treatment, let alone a clinical trial,” says Austin Kim, MD, Dos Anjos’ Dana-Farber oncologist. “He had minimal side effects, tolerated the treatment extremely well, and remains in complete remission over 20 months later. His success and the success of other patients on this trial will change the landscape of treatment in mantle cell lymphoma and allow other patients like Jose to have access to this potentially curative therapy.”
In the meantime, with his check-ups at Dana-Farber down to once every six months, Dos Anjos is enjoying walks with his wife, Estefania, and savoring his new lease on life.
“I’m so thankful to Dr. Kim and my team, and the opportunity they have given me,” says Dos Anjos. “I am very happy that the trial worked for me, and hope that more people can benefit from it as well.”
