Animals — from oysters and corals to flies and mammals — encode proteins to sense their environment and share a common strategy to rally a defense. Scientists have identified a new family of immune proteins conserved across animal evolution with common features.
Recent research from Dana-Farber Cancer Institute has added a new family to the list: cGAS-like-receptors (cGLRs). Prior to these findings, which were published in Cell, scientists were aware of only two cGLRs: the original cGAS enzyme in humans, and a cGAS-like-receptor in Drosophila, the fruit fly.
The new research uncovered about 3000 cGLRs across the animal kingdom — all with a similar structure and in the same family, but also unique and diverse from animal to animal.
“The diversity is amazing,” says senior author Philip Kranzusch, PhD, a researcher in Cancer Immunology and Virology at Dana-Farber.
The family defines a pathway in which an enzyme (such as cGAS in humans) senses pathogens and activates a protein (STING) to mount a response. In humans, this pathway is on the radar of cancer researchers because cGAS detects signs of cancer in human cells and STING responds to eliminate it.
In this paper, first author and postdoctoral fellow Yao Li, PhD, also found that there is at least one more cGLR in human cells, providing cancer researchers with more potential targets to investigate in their efforts to treat cancer.
“Before we only knew about cGAS, but now we see the full landscape,” says Li. “This is a very diverse family of pathogen-sensing enzymes that are important for the immune response to viruses and cancer.”
Adventures in the animal kingdom
To find the more than 3000 cGAS-like-receptors (cGLRs) that sense pathogens and activate STING, the team mined the National Center for Biotechnology Innovation (NCBI) database, which contains the genomes of known animals. They looked for patterns found in the genes that code for cGAS in humans and for the cGLR in the fly.
To validate and refine the search results, they selectively examined the proteins the genes code for to make sure they were structurally similar to known cGLRs. They also performed biochemical studies to make sure they had the same function as known cGLRs in vitro.
Along the way, they noticed that oysters and corals had many more cGLRs than other animals. Humans, for instance, have three. Corals and oysters have hundreds. To learn more, the team connected with scientists around the world to learn how these proteins work in marine creatures.
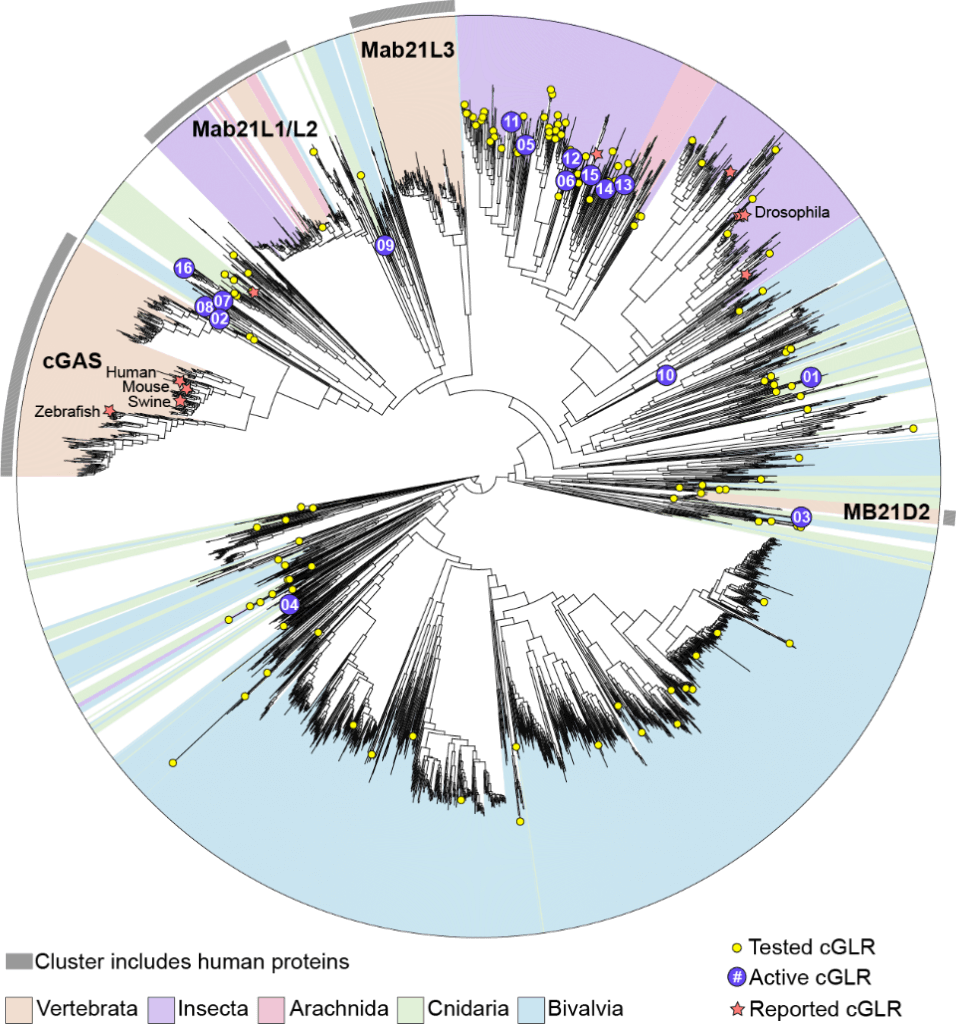
Yao speculates that corals and oysters have such a wide range of sensors because they have few other defenses. “They are filter feeders, meaning they take up nutrients and food particles by filtering the water around them,” she says. “Maybe they need a wide variety of sensors to protect them from harmful microbes.”
Ultimately, what they found has been observed time and again in biology. When evolution hits on a system that works, that system sticks around. “This signaling pathway is evolutionarily ancient,” says Kranzusch. “It’s a core principle that applies broadly to how these sensing networks work in animals.”
STINGing cancer
In humans, the cGAS enzyme is present in every cell in the body. It detects threats by detecting double stranded DNA in the cytosol, that space between the cell membrane and the nucleus.
DNA isn’t supposed to be in the cytosol. It’s only there if viruses or bacteria have entered the cell, or if DNA has leaked out from the nucleus — which can happen when cancer begins to form.
When the enzyme detects DNA, it sends out a signal in the form of a chemical called a nucleotide second messenger that activates a protein called STING. STING sounds the cell’s alarm bells. “With a little bit of activation, the cell warns its neighbors of trouble,” says Kranzusch. “But at higher levels, the cell dies.”
Researchers have shown that if you delete the STING pathway from cells, they are more susceptible to cancer. “They lose this ability to mount an immune response against cancer,” says Kranzusch.
The obvious hypothesis might be that the opposite — more STING activation — would eliminate cancer altogether. But biology doesn’t work that way. Too much STING activation results in immune responses to things that aren’t threats, such as reactions to healthy tissue, and that can result in autoimmune disease.
“Biology is about regulation,” says Kranzusch. “It’s about the correct amount of signal for the threat.”
Li and Kranzusch did not discover the correct amount of signal. What they did discover, though, is a treasure trove of new signaling molecules, STING variants, and sensing enzymes like cGAS for cancer researchers to study and learn from.
Diversity deep dive
The discovery of this family of cGLRs raises exciting new scientific questions. Kranzusch and Li are eager to understand the new human cGLR they discovered, called MB21D2. They want to know what it is sensing and how it controls the immune response.
So far, the small number of cGLRs that have been studied sense either DNA or RNA. But given the sheer number of cGLRs across the animal kingdom, some of them might sense other things. “Understanding what they sense would reveal a lot about how the immune system sees the outside world,” Kranzusch says.
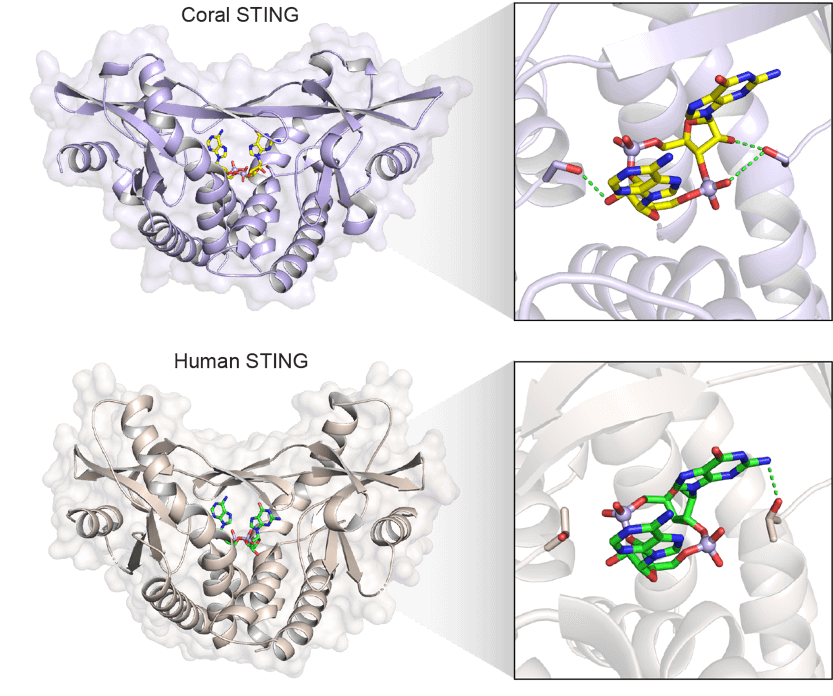
The diversity doesn’t stop with the cGLRs. STING proteins also vary widely across the animal kingdom, and even from one human to another. This research could provide insights that help drug developers find new ways to target and activate STING to treat cancer.
“Understanding how nature changed STING and the small molecules that activate it could provide insights into new ways to design cancer drugs,” says Li.
