Wind up a mechanical mouse, set it down, and watch it go. New research by Dana-Farber scientists shows that boosting a single protein in the liver can have the same animating effect in real mice.
The protein, called MIC19, produces changes deep inside liver cells that help the body burn nutrients, protecting against obesity and the development of type 2 diabetes, the researchers report in a featured article in Cell Metabolism. And, by a chain of events that leads directly to the brain, the protein acts like a motivational speaker, stirring otherwise idle mice into action and movement.
Because much of this liver mechanism is shared with humans, the findings suggest that drugs that spur production of MIC19 in liver cells could help stave off obesity and type 2 diabetes – and the diseases associated with them, including cardiovascular disease, viral infections, and some cancers – researchers say. It isn’t clear yet whether the pathway that begins with MIC19 has the same behavioral effects in humans as it does in mice, but if it does, one could imagine a future drug that prompts people to get up off the couch and do some walking — which may be all the push some people need.
“Overnutrition, especially with unhealthy foods, coupled with low physical activity, can cause a variety of metabolic diseases such as obesity and type 2 diabetes. And an overaccumulation of lipids – fatty compounds – in the liver can cause a condition known as non-alcoholic fatty liver disease [NAFLD] that can lead to liver fibrosis and liver cancer,” says Dana-Farber’s Pere Puigserver, PhD, who led the study Jee Hyung Sohn, PhD, Beste Mutlu, PhD, and Pedro Latorre-Muro, PhD, of his lab. “Expending energy, as by exercising, is an important part of controlling weight, but it isn’t always enough, on its own, to have a sufficient effect. In this study, we focused on the role of the liver in energy use.”
The liver and energy
The liver is generally thought of as the body’s filtration plant, removing toxins from the blood, but it also plays a key role in balancing the body’s energy use and keeping nutrients at stable levels. It distributes metabolites — substances made by breaking down nutrients — to tissues and organs to ensure they continue to function at the appropriate level.
The liver is as sensitive as the stomach to the presence or absence of nutrients and as sensitive as the heart to physical activity. As conditions change — as an individual begins or finishes a meal, for instance, or starts or stops exercising — the liver responds to keep bodily processes in balance.
During fasting — between meals, for example — the liver amps up the activity of its mitochondria, tiny structures that produce energy for cells. This sparks an increase in the liver’s production of glucose and other substances that power the body’s organs and tissues. This ensures that all parts of the body, including the brain, have an ample supply of fuel while food is absent.
The rise in mitochondrial activity helps ward off obesity and the development of type 2 diabetes as well as NAFLD. But the mechanism that connects food intake and fasting to changes in mitochondrial functioning were largely unknown.
Feasting and fasting
By comparing protein levels in liver cells from fasting mice and mice gorged on food, the Dana-Farber team found that MIC19 causes conspicuous changes in the structure of liver cell mitochondria.
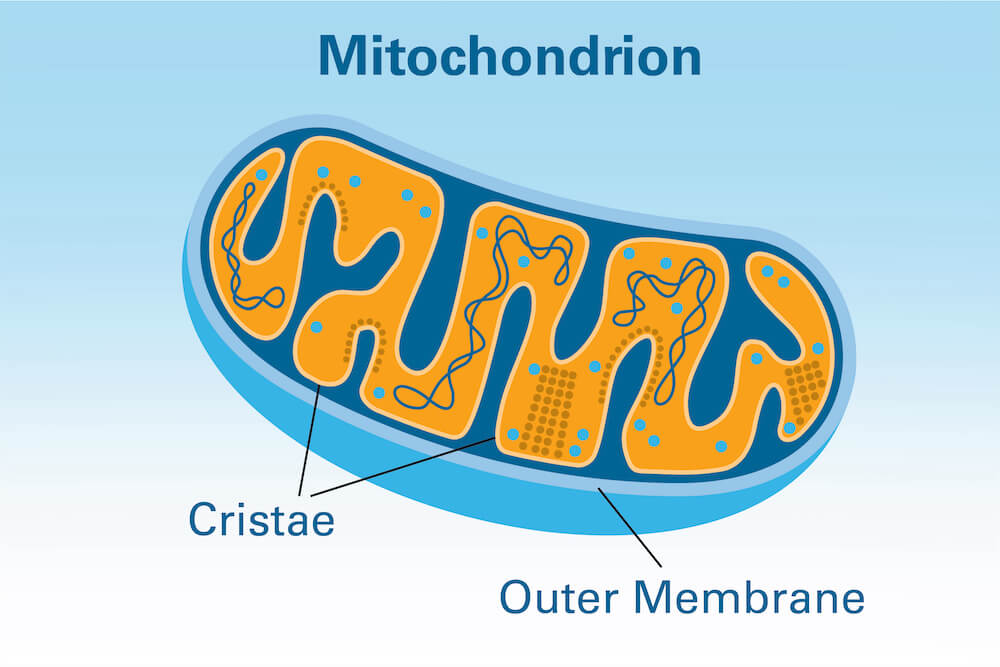
The membrane of a mitochondrion is a two-layered affair — a smooth outer membrane and an inner membrane with numerous folds called cristae that harbor respiratory machines that burn nutrients. The cristae help in the production of ATP, a compound that stores energy used by cells. The more cristae, the more fuel and energy available to cells to burn.
“We found that, during fasting, MIC19 levels in liver mitochondria rise, which promotes cristae formation,” Puigserver comments. “That, in turn, causes the liver to burn more nutrients.”
When researchers tweaked the mice’s livers to overproduce MIC19, the animals didn’t become obese even when lavishly fed and were protected against type 2 diabetes. Part of the reason they stayed slim was because they expended more energy, researchers found.
“This much was expected and logical,” Puigserver remarks. “If you promote nutrient-burning activity in the liver, the animals become leaner. What was surprising was that simply increasing MIC19 in the liver caused them to be more physically active — to walk faster and longer.
“In humans, it would be the equivalent of taking a brisk walk, not of going to the gym and exercising harder.”
The liver to brain connection
To understand how a change in metabolic activity in the liver can trigger an impulse to start walking, researchers took stock of the metabolites produced in the liver when MIC19 levels are high and found an accumulation of a compound known as uracil. “It’s likely that uracil acts in the brain to promote locomotion,” Puigserver says. Support for that theory came when researchers added uracil to the animals’ diet — and saw the animals start moving around more.
The link between MIC19 and increased metabolic activity in the liver exists in humans as well as mice, suggesting that a drug that ups MIC19 could render the liver healthier, less fatty, and less inflamed, and help deter obesity and diabetes, the study authors say. It’s unclear whether the uracil pathway acts in the human brain as it does in the mouse brain, so the notion of a drug that prods people to walk by targeting MIC19 is a bit premature, Puigserver says.
As for why fasting triggers an increase in MIC19 in the liver and thereby gets mice a-moving, Puigserver offers an evolutionary possibility. “When animals fast and there’s no food nearby, mobility is vital to the search for food. MIC19 may be part of the mechanism that sparks that search.”
