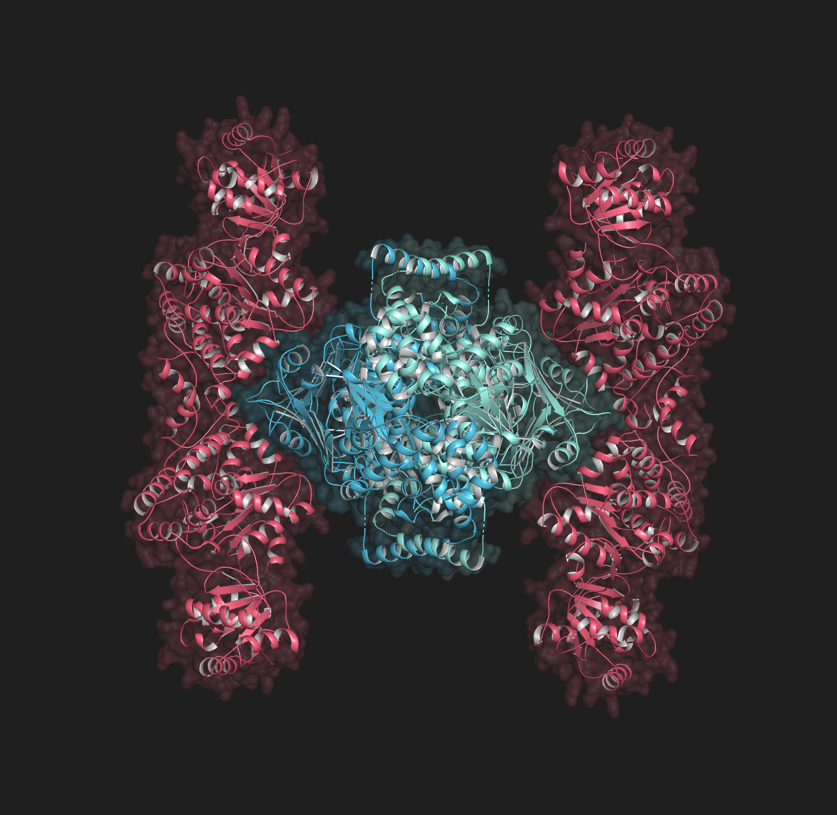
If you’ve seen the original Star Wars movie, you might wonder if the iconic Tie fighter was modeled after the strikingly similar looking Gabija protein complex, a bacterial defense system. From the right angle, they appear to share the same distinctive shape: a deadly center protected by two wings. They also share a purpose: defend the realm.
But the structure of Gabija was only revealed recently. It was determined for the first time by virology doctoral candidate Sadie Antine in the Cancer Immunology and Virology lab of Philip Kranzusch, PhD, at Dana-Farber. They published their findings in Nature.
Kranzusch and Antine study bacterial defense systems and the mechanisms that viruses that infect and replicate within bacteria, called phages, use to defeat them. They hope that by understanding the intricate details of how these systems interact, they might be better able to understand and ask questions about all aspects of immunity, including human immunity and the response to cancer.
“This is the importance of basic science,” says Kranzusch. “We’re learning how cells defend against infection.”
Snapshot of a bacterial defense system
Gabija is one of hundreds of defense systems found in bacteria. It is present in about 15% of all bacteria whose genes have been sequenced.
“It’s one of the most prevalent bacterial defense systems,” says Antine. “Yet very little was known about how it works or how viruses that infect bacteria can evade the system.”
To learn what Gabija looks like when it is a fully formed molecular machine, called a protein complex, Antine used a technique called X-ray crystallography. The process involves coaxing the bacteria to make the protein complex, crystallizing the complex so that it is immobile, and then scattering X-rays off it to get a precise, atomic-level 3D snapshot of the structure.
Gabija, she learned, is a very large complex. It is about one quarter the size of the ribosome, which is a huge molecular machine that performs the incredible task of using information from RNA to make proteins.
Antine also learned that it is formed using the instructions from just two genes, GajA and GajB. GajA forms proteins that connect in groups of four to form the center of the structure. GajB forms proteins that connect to form the outer wing-like portions of the structure.
“It’s an arrangement that you never would have guessed until you did the experiments,” says Kranzusch.
It isn’t yet clear how this large complex recognizes and defeats the phage. But Antine and Kranzusch suspect that the complex recognizes a specific structure formed by phage DNA and degrades it.
“Gabija has exquisitely evolved to hunt and destroy a very particular target,” says Kranzusch.
Evolving an evasive web
Antine and Kranzusch collaborated with a team at the Weizmann Institute in Rehovot, Israel, to home in on Gabija as a defense system of interest and to identify the phage that can evade it. With this knowledge, Antine introduced a gene identified to make phage evasive into bacteria that create Gabija to learn more about the evasion tactics.
Antine wanted to look at Gabija as it interacted with the phage protein. To do this, she used cryogenic electron microscopy (cryo-EM), which involves super cooling the complex and using a beam of electrons to create a 3D image of the system.
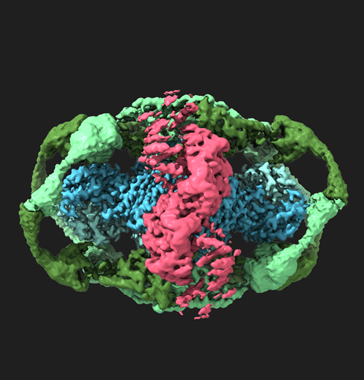
“With crystallography we see a snapshot of the protein complex in a rigid state,” says Antine. “But with cryo-EM, it is possible to analyze flexible complexes, and you can see how the interaction has played out.”
A typical evasive move would be for the phage to alter the bit of DNA that Gabija recognizes so Gabija doesn’t notice it. But the phage doesn’t do that, suggesting that what the DNA Gabija recognizes is essential for the phage to survive.
Another common tactic would be for the phage to evolve a bit of DNA that encodes a small protein that jams part of Gabija’s essential machinery. But Antine didn’t see that, either.
Rather, she found that the phage evolved DNA that encodes a very large protein that surrounds Gabija and inactivates it.
“The protein forms this huge web around the entire outside of the complex,” says Kranzusch. “This evasion technique creates a massive complex. It was a surprising result that changes the way we think about how phages interact with these defense systems.”
Entities in a molecular arms race
Phages are often thought of as small and simple, but Kranzusch has found that that’s not always true. The phages he and Antine are studying are large, with DNA that holds hundreds of genes. They are also considered “entities” rather than “living organisms” because they require a host cell to replicate. Yet they actively evolve and change under pressure from defense systems like Gabija.
“They are complex and can evolve and adapt with their host. They shape evolution,” says Kranzusch.
For next steps, Antine will dive into the precise mechanisms Gabija uses to defeat phages. These mechanisms are the result of a molecular arms race, with each side finding new ways to defeat the other. The same kind of arms race goes on in cancer, as tumor cells find increasingly clever ways to evade the immune system and cancer treatments.
“There are parallels between immunity in human cells and in bacteria,” says Antine. “We’re interested in the diversity, the many ways that immune systems combat something that is actively evolving against it.”
