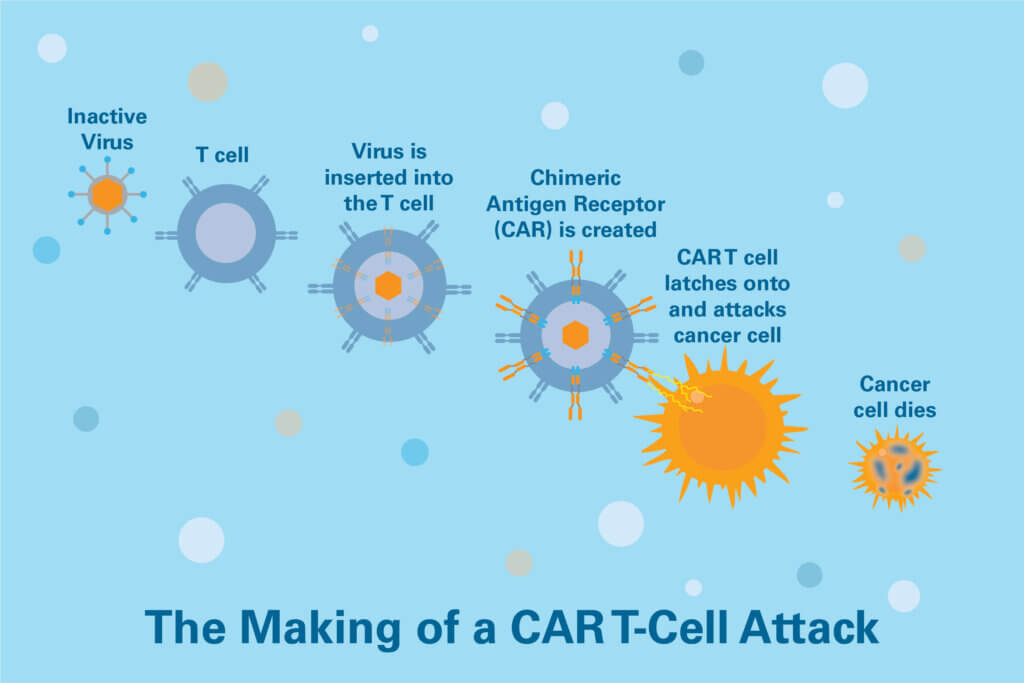
CAR T-cell therapy is a form of immunotherapy in which patients’ T cells are modified to become better at tracking down and destroying tumor cells. CAR T cells are made by extracting thousands of a patient’s T cells, sending them to a lab where they’re outfitted with genes and proteins that improve their cancer-fighting prowess, and then re-infusing them into the patient.
CAR T-cell therapy has been approved by the U.S. Food and Drug Administration as standard therapy for:
- Some adult patients with aggressive non-Hodgkin lymphoma that has relapsed after prior treatments, or has not responded to other therapies (refractory).
- Some patients with relapsed or refractory mantle cell lymphoma.
- Some patients with relapsed/refractory follicular lymphoma.
- Patients with relapsed/refractory multiple myeloma.
- Adult and pediatric patients with relapsed or refractory B-cell acute lymphoblastic leukemia.
CAR T-cell therapy can give rise to a range of side effects. As doctors have gained experience with the therapy, they’re better able to identify side effects in their earliest stages and treat them effectively. There are also treatments that can be given in advance of likely side effects to reduce their severity.
At Dana-Farber and partnering Brigham and Women’s Hospital and Boston Children’s Hospital, physicians and other clinicians have extensive experience in CAR T-cell therapy, both as standard treatment and in clinical trials. This experience enables them to provide exceptional supportive care to limit the severity of side effects and treat them effectively.
Cytokine release syndrome (CRS)
A potentially serious complication is cytokine release syndrome (CRS), also known as a cytokine storm. Once they enter the body, CAR T cells initiate a massive release of proteins called cytokines, which summon other elements of the immune system to join the attack on tumor cells. The resulting onslaught can produce dangerously high fevers, extreme fatigue, difficulty breathing, and a sharp drop in blood pressure. The condition tends to be especially severe in patients with extensive cancers.
CRS tends to arise within one to five days of the CAR T cells’ infusion into the patient, although it may occur weeks later in some cases. For most patients, the condition is mild enough that it can be managed with standard supportive therapies, such as acetaminophen and intravenous fluids.
In many cases, CRS is followed by a second wave of side effects that involve the nervous system, according to Dana-Farber’s Caron Jacobson, MD, MMSC, medical director of the Institute’s Immune Effector Cell Therapy Program and a leader of clinical trials of CAR T-cell therapy for patients with treatment-resistant large B-cell lymphomas. These problems can include tremors, headaches, confusion, loss of balance, trouble speaking, seizures, and sometime hallucinations. While the causes of the symptoms are not well understood, the symptoms generally subside in a few days, although they can last for weeks in some cases. Extreme cases may be managed with steroids.
Patients usually stay in the hospital for at least a week after CAR T-cell infusion, or until the side effects subside (generally around two weeks). Although the risk of a recurrence of symptoms is low, they do sometimes arise, so clinicians monitor patients and stay in communication with them after hospital discharge. Some CAR T-cell therapies can now be given as an outpatient procedure. Your doctor will tell if that option is right for you.
Nervous system complications
The effects of CAR T-cell therapy on the nervous system can result in symptoms such as:
- Headaches
- Changes in consciousness
- Confusion or agitation
- Seizures
- Shaking or twitching (tremors)
- Trouble speaking and understanding
- Loss of balance
Because they’re at risk for these side effects, adult patients are advised not to drive, operate heavy machinery, or do any other potentially dangerous activities for at least several weeks after getting treatment. In some cases, these symptoms can be treated with steroids and often go away within days, or sometimes longer.
Other serious side effects
Other possible serious side effects of CAR T-cell therapy can include:
- Allergic reactions during infusion of CAR T cells
- Abnormally low levels of minerals such as potassium, sodium, or phosphorous in the blood
- A weakened immune system, which increases the risk of infection
- Low blood cell counts, which can increase the risk of infections, fatigue, and bruising or bleeding
Learn more about cellular therapy for cancer from the Cellular Therapies Program at Dana-Farber.
