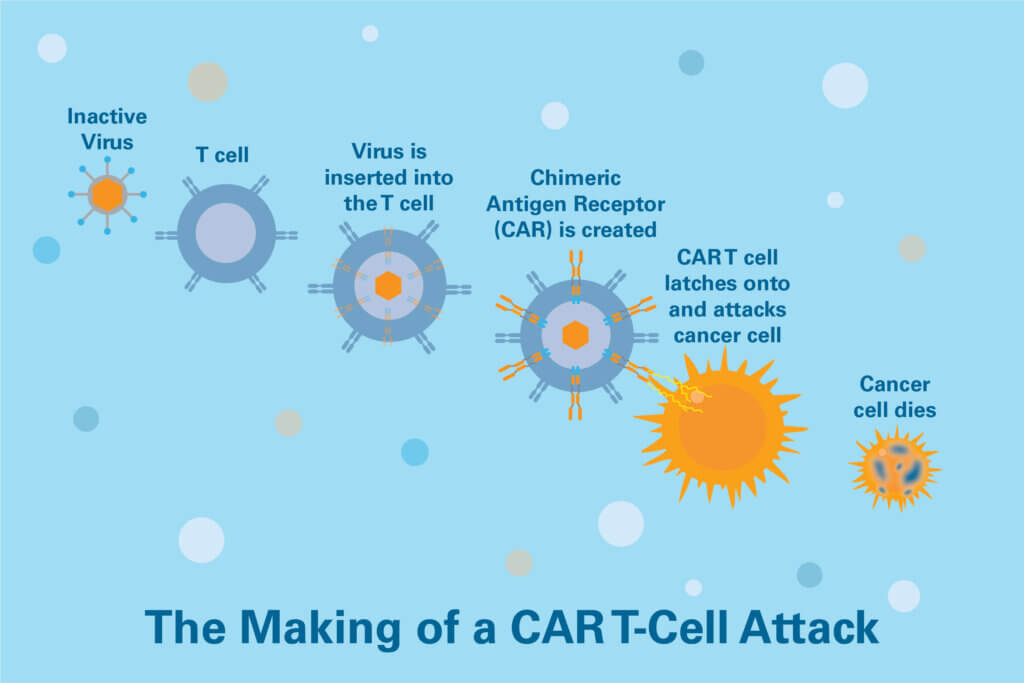
CAR T-cell therapy is a form of immunotherapy that uses specially modified T cells — part of the body’s defense system against disease — to attack cancer. It involves collecting T cells from a patient and genetically modifying them to recognize cancer cells. Reinfused into the patient, CAR T cells can spark a potent immune system response to cancer.
CAR T-cell therapy has been approved by the U.S. Food and Drug Administration as standard therapy for:
- Some adult patients with aggressive non-Hodgkin lymphoma that has relapsed after prior treatments, or has not responded to other therapies (refractory).
- Some patients with relapsed or refractory mantle cell lymphoma.
- Some patients with relapsed/refractory follicular lymphoma.
- Patients with relapsed/refractory multiple myeloma.
- Some patients with relapsed/refractory chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL)
- Adult and pediatric patients with relapsed or refractory B-cell acute lymphoblastic leukemia (B-ALL)
At the Dana-Farber Brigham Cancer Center, physicians and patient-support staff are highly experienced in delivering CAR T-cell therapy and helping patients manage side effects. The Center’s physician-researchers are also leading the way in testing the therapy in a variety of cancers.
The treatment process entails a standard series of steps.
Evaluation
Patients undergo tests and screenings to determine if CAR T-cell therapy is appropriate for them. These may include tests of:
- blood cell counts
- infection
- lung function
- heart function
- kidney and liver function
Collection
T cells are collected from patients via apheresis, a process in which blood is drawn from the body and passes through a machine that separates out T cells. The remaining blood is then returned to the patient.
Learn More:
Engineering
The T cells are sent to a laboratory where they are genetically engineered to produce chimeric antigen receptors (CARs) on their surface. CARs are proteins that allow the T cells to recognize proteins called antigens on the surface of targeted tumor cells.
Multiplication
The genetically modified T cells are grown in a laboratory until there are millions of them, which can take a few weeks. When there are enough CAR T cells, they are frozen and sent to the hospital or center where the patient is being treated.
Conditioning therapy
Prior to receiving an infusion of the CAR T cells, patients may receive chemotherapy for their cancer. This helps to create space for the infused CAR T cells to expand and proliferate in the body.
Infusion
Soon after chemotherapy, patients are admitted to the hospital and the CAR T cells are re-infused in a process similar to a blood transfusion. This is a one-time procedure, although patients may remain in the hospital for several weeks to monitor their response to the treatment, overall condition, and side effects.
Some patients may be able to receive their CAR T cells in an outpatient clinic.
Recovery and side effects
The recovery period for CAR T-cell therapy lasts approximately two to three months. After receiving their infusion of CAR T cells, patients remain in the hospital for one to three weeks so clinicians can monitor their response to the therapy and manage any side effects.
At Dana-Farber Brigham Cancer Center, some patients with lymphoma and multiple myeloma who have a low burden of disease receive their CAR T cells in the outpatient clinic. After being monitored for at least an hour they return to their “home base” — their home, hotel, or other living quarters — which must be within a 30-minute trip of the Center, and periodically monitor themselves for possible side effects of the treatment.
Patients return to the Center every day for laboratory tests and a clinical exam. These evaluations continue for a minimum of seven days. Patients must have a caregiver stay with them round-the-clock for up to a month and accompany them to hospital appointments. Patients experiencing side effects are admitted to the hospital for closer monitoring. Although the type and severity of side effects vary from patient to patient, there is a significant risk that complications will occur within a few weeks of treatment. Most often, these complications are temporary and subside on their own or with treatment. Possible side effects include:
- cytokine release syndrome, an inflammatory condition with flu-like symptoms such as high fever and/or chills but can include low blood pressure and difficulty breathing as well as other organ dysfunction
- neurologic difficulties such as confusion, difficulty understanding language and speaking, or stupor.
At the Dana-Farber Brigham Cancer Center, patient-care staff are experts in helping patients manage and minimize side effects of treatment.
After their release from the hospital, patients have periodic follow-up appointments over the next few months to be evaluated for side effects and response to the therapy. It is possible but uncommon for patients to be re-admitted to the hospital during this period with recurrent side effects.
Evaluations for how well patients respond to therapy vary with the type of cancer being treated. Patients with B-ALL have a bone marrow biopsy done a month after CAR T-cell infusion. Patients with B-cell non-Hodgkin lymphoma, large B-cell lymphoma, mantle cell lymphoma, or follicular lymphoma have a PET scan one month after treatment. A bone marrow biopsy and PET scan are used for select patients with multiple myeloma. Patients are seen by their physician every one to three months for the next several years, during which time they are monitored for disease relapse with blood tests, physical exams, and/or bone marrow biopsies and PET or CT scans.
Because each patient is different, patients should talk with their physician about each aspect of their treatment and raise any concerns they may have.
