Follicular lymphoma is a slow-growing form of non-Hodgkin lymphoma (NHL) that arises in white blood cells called B lymphocytes. It’s named for the clumps of abnormal B cells, called follicles, that develop in patients’ lymph nodes. It accounts for 20-30% of all cases of NHL.
Although follicular lymphoma currently isn’t curable, patients can live with it for many years, making it more of a chronic disease.
Treatment for follicular lymphoma is geared to each patient’s situation. If the disease is confined to one area, radiation therapy may be recommended. If a patient is free of symptoms, doctors may advise a “watch and wait” approach in which the patient is monitored closely but not treated. When symptoms do develop, the standard treatment has been to combine chemotherapy with an antibody drug that targets the CD20 protein on follicular lymphoma cells. That approach usually succeeds at putting the disease in remission, but the chemotherapy can trigger a range of adverse side effects, particularly among older patients, who constitute the majority of patients with follicular lymphoma.
New approaches to treatment
As researchers have learned more about the basic biology of follicular lymphoma, and of the immune system’s response to the disease, they’ve developed potential treatments that may offer an alternative to chemotherapy. At Dana-Farber, investigators are leading clinical trials of several classes of new agents:
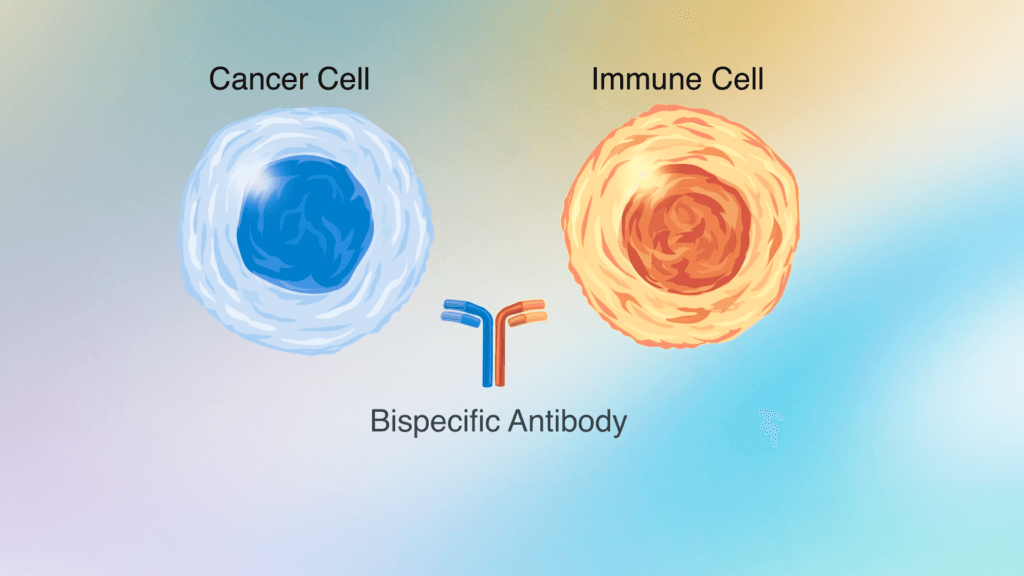
Bispecific antibodies
Bispecific antibodies are agents that simultaneously target tumor cells and immune system cells, helping to spur an immune attack on the cancer. In clinical trials, bispecific antibodies that bind to the CD3 protein on T cells (an immune cell) and to CD20 on follicular lymphoma cells produced positive responses in about 80% of patients with follicular lymphoma, including many who had already received multiple treatments for the disease. Side effects of the treatment were largely manageable. Dana-Farber investigators are now leading two phase 2 trials that combine a bispecific antibody with a monoclonal antibody that targets CD20 alone:
- A trial of the monoclonal antibody rituximab plus the bispecific antibody epcoritamab
- A trial of the monoclonal antibody obinutuzumab plus the bispecific antibody glofitamab

In both trials, patients initially receive the CD20 monoclonal antibody to reduce the overall burden of lymphoma, followed by the bispecific antibody. Once patients start the bispecific antibody, they are monitored closely for a common side effect known as cytokine release syndrome (CRS), in which the immune system mounts too aggressive an attack on the cancer. To reduce the risk of severe CRS, which usually arises early in the course of treatment, physicians gradually increase the dose over the first three weeks of treatment. By giving the monoclonal antibody drug first, researchers hope to further lower the risk of CRS.
In conjunction with the trials, researchers are collecting tumor samples at the start of treatment as well as blood and stool samples over the course of therapy to look for markers that indicate whether a patient is likely to do well and whether he or she is likely to develop CRS.
Trials for patients whose disease has relapsed within 24 months
Dana-Farber researchers are leading two trials for patients whose cancer has relapsed within the first two years of treatment, who tend to have worse outcomes than patients whose follicular lymphoma does not relapse as quickly.
- A trial of epcoritamab plus the immune-stimulating drug lenalidomide and rituximab
- A phase 3 trial comparing the standard therapy for relapsed follicular lymphoma — chemotherapy or lenalidomide and rituximab — to CAR T-cell therapy, which uses genetically modified versions of a patient’s own immune system T cells to attack cancer.
Trials for patients with later relapse
Dana-Farber investigators are also leading trials for patients whose follicular lymphoma relapses outside the two-year window.
- A trial of a novel type of cancer vaccine. The immune system is constantly in contact with bacteria in the digestive tract and some of these bacteria have antigens — surface markers that identify cells to the immune system — similar to those found on follicular lymphoma B cells. In this trial, a vaccine was developed that prompts the immune system to attack lymphoma cells carrying these antigens. The trial is open to two groups of patients. Those whose follicular lymphoma is resistant to previous treatment or has relapsed will receive the vaccine plus the lenalidomide/rituximab combination and those who haven’t developed symptoms or received previous treatment will receive the vaccine by itself.
“We are thrilled to have multiple clinical trials testing promising, novel immunotherapy approaches for patients with follicular lymphoma, and we are hopeful that these trials can lead to a future were chemotherapy is no longer necessary for many patients with the disease,” says Reid Merryman, MD, of the Lymphoma Program at Dana-Farber.
About the Medical Reviewer
Dr. Merryman received his undergraduate degree from the University of Notre Dame and his medical degree from Harvard Medical School. He completed residency training in internal medicine at Brigham and Women's Hospital and fellowship training in hematology and oncology at Dana-Farber/Partners Cancer Care. He is an attending physician in the Lymphoma Program at Dana-Farber Cancer Institute. His research focuses on the development of immune-based therapies for patients with lymphoma.

This was a very informative article. As a follicular lymphoma (FL) patient who was in remission for ~6 years and now has indications that likely represent early recurrence of FL, I hope you will issue a follow-up to this article to provide feedback about the trial findings and any other new trials and treatments for FL. Thank you.