Gene therapy is a way of treating or preventing disease by altering the genetic instructions within an individual’s cells. Genes are responsible for virtually every aspect of cell life: they hold the code for proteins that enable cells to grow, function, and divide.
When a gene is defective, it can give rise to proteins that are unable to do their job or no protein at all. When a gene is missing, or is overactive, important bodily functions may be impaired. One approach to gene therapy is to correct such problems by fixing them at the source.
This type of gene therapy, called in vivo gene therapy, uses a virus or a gene editing tool such as CRISPR inside the body of the patient to correct a genetic abnormality in specific cells.
- One adenovirus-based gene transfer therapy that introduces a gene into bladder cells is approved for high-risk, non-muscle invasive bladder cancer.
- There are several approved gene therapies for non-cancerous diseases caused by a single gene mutation. In 2023, the U.S. Food and Drug Administration (FDA) approved CRISPR-based gene therapy to correct the gene abnormality that causes sickle cell disease. Dana-Farber’s Stuart Orkin, MD, was the first to conceive of this genetic correction and the possibility of gene therapy for the disease.
Cancer researchers, including experts at Dana-Farber, have also devised a variety of creative gene therapies. Some involve altering the genes of a patient’s immune cells outside the body and reinfusing them, known as ex vivo gene therapy. Others involve genetically engineering viruses to fight cancer. For example:
- CAR T-cell therapy alters immune cells called T cells. Six CAR T-cell therapies are currently FDA approved for the treatment of certain blood cancers. There are hundreds of CAR T-cell trials running across the globe in many types of cancer, including solid tumors.
- CAR NK-cell therapy alters immune cells called natural killer (NK) cells. CAR NK-cell therapies are not yet approved for the treatment of cancer but are being tested in clinical trials.
- Therapeutic cancer vaccines alter antigen-presenting immune cells. The first therapeutic cancer vaccine to receive FDA approval is used in the treatment of certain prostate cancer patients.
- Oncolytic viruses are engineered viruses that directly kill cancer cells. An oncolytic virus therapy is approved for the treatment of advanced melanoma.
How do gene therapies work?
In vivo gene therapy: The intention of in vivo gene therapies is to use viral vectors or CRISPR gene editing tools to make genetic alterations. These tools can introduce, inactivate, or fix a gene. Genes can be altered in cancer cells or in surrounding tissue. The goal is that the newly inserted gene will cause the cancer cells to die or interrupt some process required for survival, such as preventing cancer cells and surrounding tissue from funneling blood to tumors and depriving them of nutrients.
While this approach has a great deal of promise, it presents scientists with several obstacles, including “gene silencing,” in which the implanted genes fail to switch on. In animal studies, gene transfer techniques achieved positive results in treating prostate, lung, and pancreatic tumors.
Various approaches to gene transfer are being tested in clinical trials. These trials have involved cancers including blood cancer and cancers of the head and neck, liver, ovaries, prostate, bladder, and other organs.
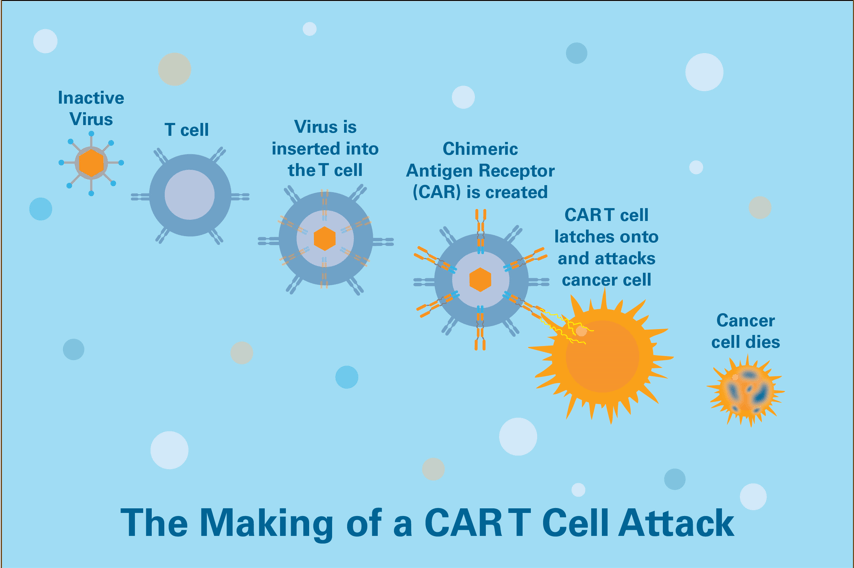
CAR T-cell therapy: Although there are many varieties of CAR T cells, most commonly a sample of a patient’s own immune T cells is collected and mixed with viruses carrying one or more specific genes. The viruses deliver these gene(s) to the T cells’ nuclei, where they’re incorporated into the cells’ DNA.
The gene causes the T cells to express a special protein called a chimeric antigen receptor, or CAR, on their surface. The CAR directs the T cell to a specific “address” present on tumor cells. When the CAR T cells are infused into the patient, they seek out tumor cells and then proliferate to generate many more cancer-killing CAR T cells. Patients receiving CAR T-cell therapy are monitored closely because this rapid cell expansion can overstimulate the immune system and cause an inflammatory response called a cytokine storm.
CAR T-cell therapy is currently approved for the treatment of:
- B-cell acute lymphoblastic leukemia (ALL)
- B-cell non-Hodgkin lymphoma including
- Large-B-cell lymphoma
- Follicular lymphoma
- Mantle cell lymphoma
- Multiple myeloma
Efforts are underway to create off-the-shelf CAR T-cell therapies derived from healthy donors that do not require the collection and engineering of a patient’s own cells.
There are several CAR T-cell therapy trials available for blood cancers and solid tumors in adults and also there are some trials that will open soon for pediatric cancers.
CAR NK-cell therapy is very similar to CAR T-cell therapy, except it involves genetically altering immune cell called natural killer (NK) cells. Because NK cells act differently than T cells, CAR NK-cell therapy is less likely to overstimulate the immune system.
The research of Dana-Farber’s Rizwan Romee, MD, focuses on finding ways to make NK cells more active and better at fighting cancer using a variety of techniques, including genetic engineering.
CAR NK-cell therapy is currently being tested in clinical trials for many forms of cancer including leukemia, lymphoma, multiple myeloma, ovarian cancer, colorectal cancer, and pancreatic cancer.
Therapeutic cancer vaccines: These vaccines, also called tumor vaccines, teach the immune system to better recognize cancer cells in the body.
One type of cancer vaccine is a cellular therapy. This type of therapy typically alters a patient’s immune cells known as antigen-presenting cells so that they better display a protein known to identify the cancer. When reinfused into the patient, these engineered cells can train the patient’s T cells to better recognize and thus fight the cancer.
Another type of cancer vaccine is a protein-based vaccine, which can be personalized for each patient since each person’s cancer will have a unique signature. Investigators Patrick Ott, MD, PhD, and Catherine Wu, MD, at Dana-Farber’s Center for Personal Cancer Vaccines, are leading clinical trials of a protein-based vaccine called NeoVax in patients with melanoma, glioblastoma brain cancer, and kidney cancer.
Oncolytic viruses: Genetically engineered viruses can target and destroy cancer cells while leaving normal cells unharmed. Such oncolytic viruses are designed to infect cancer cells and, once inside, to produce proteins that cause the cells to die.
A variety of oncolytic virus therapies are being tested in clinical trials for malignant glioma and glioblastoma (brain cancers), and a range of metastatic solid tumors.
Learn more about cellular therapies in trials at Dana-Farber.
Learn more about immunotherapy at Dana-Farber.
How is gene therapy made?
Gene and cell therapies require special expertise to manufacture. The procedures may involve genetic engineering techniques, collection of cells (often from the patient), and specialized cell handling, processing, and packaging methods.
Dana-Farber’s Connell and O’Reilly Families Cell Manipulation Core Facility (CMCF) plays a central role in advancing cell and gene therapies for cancer and prepares many genetically modified and non-modified cellular therapies for patients at Dana-Farber and elsewhere.
The CMCF is among the largest of academic cell therapy manufacturing facilities, with a staff of over 70 people. The team supports more than 80 clinical research protocols, manufactures novel products for over 20 US Food and Drug Administration (FDA) investigational new drug (IND) applications, and dispenses over 1400 cellular products each year. The facility’s manufacturing facilities support approved and experimental cellular therapies.

Does Dana Farber offer gene sequencing of tumors similar to that available at Memorial Sloan Kettering with its MSK-IMPACT program, which sequences over 400 genes?
Please advise.
Thank you.
Hi Bert,
Apologies for the delay in response. Profile, a research project launched by scientists at Dana-Farber Cancer Institute and Brigham and Women’s Hospital, is one of the nation’s most comprehensive personalized cancer medicine initiatives. Since the project began in 2011, Profile scientists have been analyzing the DNA in tumor tissue from patients coming to Dana-Farber Cancer Institute and Brigham and Women’s Hospital for treatment of all types of cancers. Here is where you can find more information: http://www.dana-farber.org/research/featured-research/profile/
Wishing you the best,
DFCI