Immunotherapy refers to treatments that use the body’s immune system to combat diseases. Immuno-oncology focuses on efforts to use the immune system as a weapon against cancer.
The immune system is a collection of organs, tissues, specialized cells, and substances that protect the body against infection and disease. While the immune system can often handle very small tumors on its own, either by destroying them or keeping them from growing, it can sometimes fail — either because a tumor grows too large, the cancer cells are too well camouflaged, or the tumor cells are able to stave off an immune system attack.
A key discovery, made by Dana-Farber’s Gordon Freeman, PhD, and others, was that the immune system’s attack on cancer often falls short because many cancer cells display proteins, called immune checkpoint proteins, that bring the attack to a halt. Specifically, Freeman discovered the immune checkpoint protein PD-L1 and its interaction with another immune checkpoint protein called PD-1.
Many experts from Dana-Farber and elsewhere have contributed to turning these and other discoveries about the immune system into immunotherapies for cancer. Over the past decade, immunotherapy has fundamentally changed the way cancer is treated in many patients.
Today, there are several types of immunotherapies for cancer:
- Some help train the immune system to better recognize and attack cancer cells.
- Others harness specific agents of the immune system, such as antibodies, to become cancer fighters.
- Still others work by releasing the molecular “brakes” on the immune system’s attack on cancer.
Dana-Farber researchers are focused on continuing to make advancements, setting the stage for an even more extensive role in cancer treatment in the future.
Types of immunotherapy
Cancer vaccines
Cancer vaccines are substances given to people to prevent cancer from developing, or to treat existing cancers by strengthening and optimizing the body’s immune response against the tumors.
An example of a preventive vaccine is one that protects against infection with the human papilloma virus (HPV), which causes cervical cancer and some other cancers, including cancers of the mouth and throat.
The first therapeutic cancer vaccine was approved by the U.S. Food and Drug Administration in 2010. Called Provenge, it is designed to stimulate an immune response against metastatic prostate cancer. Provenge is customized to each individual patient. The pivotal clinical trials leading to the approval of Provenge were done at Dana-Farber.
In addition, Dana-Farber researchers are testing experimental treatment vaccines in a variety of cancers, including:
- Melanoma
- Brain tumors
- Breast cancer
- Kidney cancer
- Leukemia
Cancer vaccines can also be combined with other types of therapies.
Monoclonal antibodies
Monoclonal antibodies are synthetic copies of antibody proteins that exist in the immune system. Their job is to identify foreign invaders by binding to specific proteins called antigens, which dot the surface of cells. After they bind, antibodies recruit other cells and substances of the immune system to attack the foreign cells.
Researchers can make large numbers of identical (monoclonal) synthetic antibodies in the laboratory and give them to cancer patients. Monoclonal antibodies are used as checkpoint inhibitors to block checkpoint proteins that the cancer cells are using to evade an immune system attack. Other monoclonal antibodies attach to and block proteins that appear on the surface of cancer cells and help the cells grow and spread.
Bispecific antibodies that block two checkpoints simultaneously are also emerging. Today, one bispecific antibody is approved by the U.S. Food and Drug Administration for certain patients with acute lymphoblastic leukemia (ALL), but they are showing promise in early trials for patients with other types of leukemia, non-Hodgkin lymphoma, and multiple myeloma. For example, a bispecific antibody that blocks both the PD-1 and TIM-3 checkpoints is in clinical trials at Dana-Farber for patients with relapsed or refractory Hodgkin lymphoma.
The power of antibodies to treat cancer has expanded with the advent of agents called antibody-drug conjugates (ADCs), which consist of monoclonal antibodies bonded to chemotherapy drugs. The antibody carries a potent chemotherapy agent directly to cancer cells, maximizing chemotherapy’s effectiveness.
Dana-Farber’s Ursula Matulonis, MD, led clinical investigations of the drug mirvetuximab soravtansine for ovarian cancer that led to its approval in late 2022. It was a significant milestone, marking the first new therapy for platinum-resistant ovarian cancer in nearly a decade.
Antibody drug conjugates are currently approved for:
- Metastatic breast cancer
- Gynecologic cancers
- Leukemias
- Lymphomas
- Urothelial cancers
- Head and neck cancers
- Multiple myeloma
- Gastric carcinoma
CAR T-cell therapy
Another exciting entry into the immunotherapy world, known as CAR T-cell therapy, has had success in some blood cancers and is being tested in trials with other hard-to-treat cancers. CAR stands for chimeric antigen receptor. CAR T cells are T cells that have been extracted from the patient’s blood and “trained” in the laboratory to recognize and kill tumor cells. They’re then returned to the patient as supercharged cancer-killers.
Dana-Farber clinicians have a long history of advancing novel CAR T-cell therapies. For instance, Dana-Farber’s Caron Jacobson, MD, was a lead investigator in pivotal trials that led to the approval of CAR T-cell therapy for certain forms of lymphoma. Prior to joining Dana-Farber, Eric Smith, MD, PhD, helped identify a new target for CAR T-cell therapy for multiple myeloma, GPRC5D, and continues that work here both in his lab and in clinical trials.
Today, CAR T products have been approved by the FDA for some forms of lymphoma and leukemia and for multiple myeloma. Dana-Farber clinicians offer clinical trials of CAR T-cell therapies in several blood cancers and in some solid tumors.
Patients with certain blood cancers have experienced remarkable remission rates after CAR T-cell therapy, though CAR T-cell therapies can overstimulate the immune system and cause significant side effects. Clinicians at Dana-Farber Brigham and Women’s Cancer Center (DF/BWCC) and Dana-Farber/Boston Children’s Cancer and Blood Disorders Center have built an extensive infrastructure to ensure the therapy is delivered safely.
Immune checkpoint inhibitors
Drugs known as immune checkpoint inhibitors prevent tumor cells from evading immune system attackers. These medicines have had striking success in many types of cancer.
Tumor cells can evade the immune system by sending a “stand down” message to T cells. This signal halts anti-cancer attack by exploiting an immune checkpoint, a mechanism intended to keep the immune system in check.
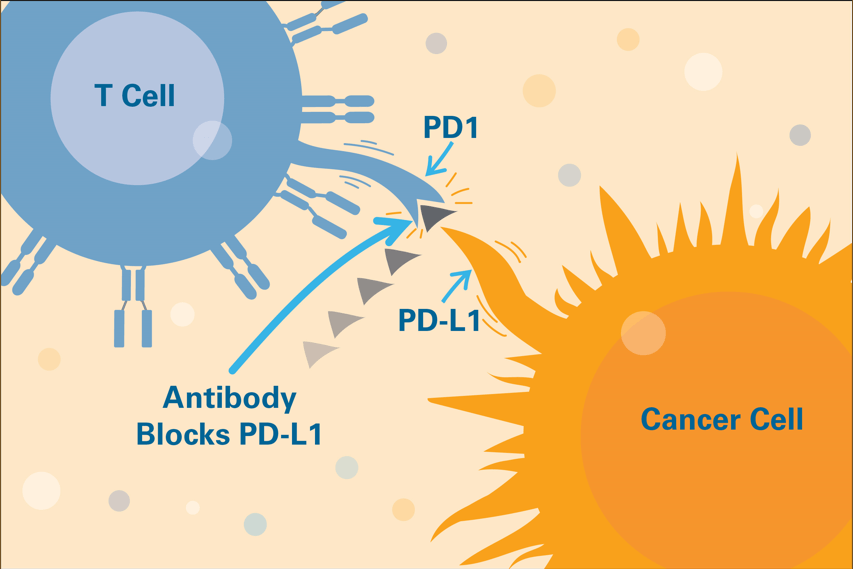
The FDA has so far approved immune checkpoint inhibitors that interrupt four of these checkpoint signals: PD-1, PD-L1, CTLA-4, and LAG-3. Freeman’s discovery of PD-L1 and the PD-1/PD-L1 pathway helped make some of these checkpoint inhibitors possible.
These medicines have been approved for use in many forms of cancer, notably:
Hodgkin lymphoma
In Hodgkin lymphoma (HL), for example, a clinical trial led by Dana-Farber’s Margaret Shipp, MD, and Philippe Armand, MD, PhD, found that the checkpoint inhibitor nivolumab drove the disease into full or partial remission in 69 percent of patients, all of whom had drug-resistant forms of the disease. The findings led the Food and Drug Administration to approve the drug for patients with classical HL that has come back or progressed after a stem cell transplant.
Melanoma
Clinical trials led by Dana-Farber’s F. Stephen Hodi, MD, showed that the checkpoint inhibitor ipilimumab could slow advanced melanoma in a significant number of patients and prolong their survival. The trial marked the first time a drug — any drug — had improved survival rates for patients with advanced melanoma.
Checkpoint inhibitors have shown exceptional promise in laboratory studies and clinical trials involving:
- Glioblastoma brain cancer
- Kidney cancer
- Lung cancer
- Bladder cancer
- Other malignancies
One of the most exciting aspects of checkpoint inhibitor drugs is that, although they tend to work in a relatively small percentage of patients with certain cancers, when they do work, their effects are often lasting.
Additional ICIs are under investigation, such as agents that block a checkpoint called CD-47, and agents that block more than one ICI at a time, called bispecific antibodies.
Combination therapy
Recent trials have shown that combining checkpoint inhibitors with other drugs can produce impressive results, particularly in types of cancer that don’t respond to checkpoint inhibitors alone. A Dana-Farber-led trial found that pairing a checkpoint inhibitor with a drug known as a PARP inhibitor was significantly more effective than either drug alone in women with hard-to-treat ovarian cancer.
Approved combination therapies include an approved ICI plus:
- Chemotherapies
- PARP inhibitors
- Other targeted therapies, including those that inhibit the growth of new blood vessels
- Monoclonal antibody therapies
ICIs are also combined with one another. For instance, two ICIs, ipilimumab and nivolumab, are approved in combination for metastatic melanoma, metastatic non-small cell lung cancer, colorectal cancer, and more.
Other potential combination partners for ICIs include:
- Radiation therapy
- CAR T therapy
- Additional targeted therapies
- Antibody-drug conjugates
- Cytokines, chemicals used by the immune system to suppress or stimulate the immune system
- Novel immune checkpoint inhibitors
Current research
Immunotherapies have changed the game for many patients with cancer, but challenges remain. CAR T therapy, for instance, can cause significant immune reactions in some patients. Further, CAR T therapies haven’t yet proven to be effective for patients with solid tumors.
In addition, immune checkpoint inhibitors have provided many patients with durable remissions for cancers that had been difficult to treat otherwise. But not everyone responds to these therapies.
Researchers at Dana-Farber are focused on finding ways to increase the numbers of patients who respond to ICIs and on devising novel immunotherapies.
Increasing numbers of responders through combinations
Some patients have tumors that are considered “hot.” These tumors are more likely to provoke an immune system to rally T cells to attack on the cancer. Some, however, are “cold,” and T cells are stymied by a range of mechanisms.
A major focus of current research involves finding ways to make “cold” tumors immunologically “hot” by combining immune checkpoint inhibitors with other therapies in ways that generate a more potent and precise attack on cancer.
Investigators are exploring several such combinations:
- Hodi led early clinical studies involving two-part therapy combining checkpoint inhibitors with other interventions in patients with melanoma. Today, many clinical studies involve various combinations of checkpoint inhibitors as well as combinations of checkpoint inhibitors with other medicines, such as drugs that block the formation of blood vessels.
- Researchers are increasingly coming to recognize that drugs designed to incapacitate cancer cells from the inside can also spark an immune response that menaces them from the outside.
- Dana-Farber researchers have shown that drugs known as CDK4/6 inhibitors, which undermine cancer cells by halting their division, can spark an immune assault on the cells as well.
- Scientists led by Dana-Farber’s Geoffrey Shapiro, MD, recently showed that targeted drugs known as PARP inhibitors, which hamper cancer cells’ ability to repair damage to their DNA, can also incite an immune system response to the cancer.
Cancer vaccines, NK cells, cytokines, and more
Researchers are also devising creative new approaches to immunotherapy.
Personalized cancer vaccines
Dana-Farber’s Catherine Wu, MD, and Patrick Ott, MD, PhD, have tested a novel approach to a cancer vaccine. This approach, called NeoVax, is created using pieces of cancer-related proteins from patients’ tumors. When injected into patients, these pieces of proteins — which are personalized and unique to each patient — spur T cell responses.
NeoVax is being tested in clinical trials for several other forms of cancer including renal cell carcinoma, glioblastoma multiforme, ovarian cancer, melanoma, chronic leukemia and lymphoma, and follicular lymphoma.

Natural Killer (NK) cells
Similar to T cells, NK cells have the power to fight cancer cells, though they don’t have the ability to learn to seek them out for destruction. Prior to joining Dana-Farber, Rizwan Romee, MD, discovered a way to give NK cells memory-like qualities that enable them to learn what cancer cells look like and seek them out.
He and others have also found ways to genetically engineer CAR NK cells, which behave like CAR T cells, but without the risk of overstimulating the immune system.
NK cell therapy is being tested in many forms of cancer, including leukemia, lymphoma, multiple myeloma, ovarian cancer, colorectal cancer, and pancreatic cancer.
Potential side effects
Like virtually all cancer therapies, immunotherapies carry the potential for side effects. Such effects vary depending on which type of cancer a patient has, how advanced it is, the type and dosage of immunotherapy used. The most common side effects are skin reactions at the needle site:
- Pain
- Swelling
- Soreness
- Itchiness
- Rash
Some patients experience flu-like symptoms, such as:
- Fever
- Chills
- Weakness
- Dizziness
- Nausea or vomiting
- Muscle or joint aches
- Fatigue
- Headache
- Changes in blood pressure
Other effects can include:
- Swelling and weight gain from retaining fluid
- Heart palpitations
- Sinus congestion
- Diarrhea
- Risk of infection
- Organ inflammation
Some of these symptoms result when the immune system, mobilized to fight cancer, mistakenly attacks health, normal tissue.
Most immunotherapy-related side effects appear in the first few weeks or months of treatment. With proper management, they generally lessen or go away in a few weeks.
About the Medical Reviewer

Dr. Hodi is the Director of the Melanoma Center and the Center for Immuno-Oncology at Dana-Farber Brigham Cancer Center and Professor of Medicine at Harvard Medical School. He received his MD degree from Cornell University Medical College in 1992. Dr. Hodi competed his postdoctoral training in Internal Medicine at the Hospital of the University of Pennsylvania, and Medical Oncology training at Dana-Farber cancer Institute where he joined the faculty in 1995. His research focuses on gene therapy, the development of immune therapies, and first into human studies for malignant melanoma. Dr. Hodi is a member of the National Comprehensive Cancer Network, the American Society of Clinical Oncology, the Eastern Cooperative Oncology Group Melanoma Committee, the International Society for the Biological Therapy of cancer, and a founding member of the Society for Melanoma Research.

THANKS FOR THIS IMPORTANT INFORMATION !!!!!
The article is very good, educational and clear, on an exciting subject. Thank you !
Enjoyed this! My wife was a participant in clinical trials with ipilimumab approximately 9-10 years ago. It prolonged her life by one year beyond the original prognosis. At that time she was hoping that others would benefit by her participation.
We have benefited! Thank you for your continued interest and your sacrifices as a care giver. So wonderful that your wife enjoyed one more year, a blessing and a gift.
Thanks for very good information
I had advanced melanoma with metastasis to brain and bowel in 2010. My oncologist strongly suggested that I get on immunotherapy medicine Leukine. That was in 2010, my health is excellent, still on the drug.
We are trying immunotherapy for my multiple myeloma. Which is hopefully working
I’m interested in any new treatments and/or clinical trials related to liver cancer. Thanks.
Car T Cell Therapy at Dana Farber has put me into complete remission for over a year now from Follicular NHL. A real life saver.
I was just enrolled in a Phase 1 clinical trial for colon cancer, MSS with zero genetic mutations, for immunotherapy using a two-drug combination. One is supposed to make the tumor “hot” like the article talks about and the other is the immunotherapy drug. I will know by next week if I am accepted. The trial is testing a multitude of cancers, not just CRC.
Drugs used:
Cabozantinib
Atezolizumab
I am stage IV melanoma survivor whose life was saved by IPI and Nivo. Thank you Dr Hodi.