There’s a critical moment in zombie movies, says Judith Agudo, PhD, a principal investigator in the Cancer Immunology and Virology Department at Dana-Farber Cancer Institute. “Someone gets bitten, and they know they’re going to turn into a zombie. They’ve still got a chance to tell their friends to kill them before they become a zombie too.”
Cancer has a similar moment. When something starts to go wrong inside a cell in the colon, that cell can send out an alert before it becomes cancerous, and the immune system will respond and destroy it.
The system works incredibly well. Except for when it doesn’t work at all, and cancer forms.
A new study co-directed by Agudo and Omar H. Yilmaz, PhD, at MIT, has found a signal that short circuits the alarm system in stem cells in the colon and enables the cells to evade the immune system and develop into cancers in animal models. The work, published in Nature, is the first to look deeply at molecular changes that protect cancer cells from surveillance by the immune system at the earliest stages of cancer development, when the cells are most vulnerable to immune detection.
“What happens between the very first mutation and the development of cancer is a mysterious process,” says Agudo. “I’m really interested in it because if we can understand it, we could potentially find ways to prevent cancer or stop it early. Can you imagine? That would be so powerful.”
Early discoveries
Colon cancer is on the rise, particularly in young people. When the cancer advances, it often doesn’t respond to immunotherapies, which have been successful in the treatment of other cancers. Agudo wanted to understand why.
As a postdoc, she’d studied how the immune system interacts with intestinal stem cells, called LGR5+ cells, which continuously replenish the lining of the colon. “It’s constant turnover,” she says. “The cells you have today in your colon will not be the cells you have next week.”
LGR5+ cells also give rise to colon cancer. In studies in her lab, Agudo knew that these cells are vulnerable to recognition by the immune system, so immune cells would clear these stem cells when they showed early signs of trouble that might lead to cancer. But not always.
“People still get colon cancer,” she says. “How are these cells able to rewire themselves to escape the immune system? Only the ones that can hide from immune recognition will grow into a tumor.”
To learn more, Agudo partnered with colleagues at MIT who were working with LGR5+ stem cells. They took the cells from mice, edited them to contain a few key mutations known to be common in colon cancer, and then reinserted them back into the mice.
In some of the mice, the cells were killed by the immune systems, says Agudo. “We never got those back. But the ones that were able to rewire and hide from the immune system, they grew into cancer.”
The team harvested these cells and used multiple molecular sequencing tools to determine what had changed in genetic wiring of these cells.
One culprit stood out: a transcription factor called SOX17. Transcription factors are proteins that regulate other genetic programs. In the cells that went on to become cancerous, SOX17 had gone from being turned off, to being turned up.

Fetal program: ON
SOX17 orchestrates a genetic program that is only supposed to be turned on during fetal stages of development. The fetus forms organs quietly, without stimulating immune attack, and SOX17 may be part of that process.
But when SOX17 is turned on in an adult, it hampers the cell’s ability to sound alarms when things go wrong. The cells change from highly immune-susceptible LGR5+ cells to SOX17 cells that cloak from immune recognition.
Agudo and colleagues don’t yet know how SOX17 is turned on, but they were able to uncover how SOX17 changes the cells.
The SOX17 program dampens a cells ability to sense interferon-gamma, a chemical that the immune system uses to communicate with cells and detect danger. The SOX17 program also hampers the cell’s ability to raise warning flags, in the form of MHC-1 molecules, on its cell surface. Without these methods of communication, the immune system isn’t called in to attack.
“The cells are in a fetal state that is very immunologically silent,” says Agudo.
To determine if the findings were relevant to human cancers, the team investigated biopsies of human precancerous polyps called adenomas. In 100% of the adenomas they studied, SOX17 was turned on, even though it should never be turned on after birth.
Fetal program: OFF
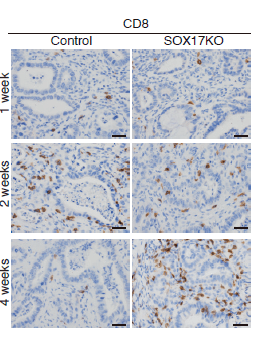
Agudo and colleagues wanted to understand what would happen if SOX17 wasn’t part of the cellular instruction set and could not be turned on. They edited the cells to knock out SOX17 and performed their experiments again, removing colon cells, introducing mutations, and implanting them back into the mice.
This time, only 6% of the implanted cells became cancerous, compared to 80% when SOX17 was present. Of those that became cancerous, the tumors remained small and were infiltrated with immune cells.
“I was shocked to see how well the immune system could kill these tumors,” says Agudo. “We had this amazing T cell response with lots of activated T cells infiltrating the area. The immune response was incredible.”
The finding suggests that the immune system can detect and eliminate cells at the earliest stages of abnormalities that might lead to cancer. There is an important moment of opportunity – just like with zombies – to intervene, stop SOX17, and expose these abnormal cells to the immune system before they become cancerous.
That moment is likely fleeting. Once a cancer develops and advances, SOX17 is less frequently turned on. By then, the cancer has accumulated even more mutations and has created an immunosuppressive environment to live in. The SOX17 program is no longer needed.
This prediction was supported by their studies of human colon cancer samples. SOX17 was turned on only in 70% of human colon cancer samples they examined.
“This correlates with what we had seen in mice, which is that SOX17 is essential at early stages, when tumor cells are unprotected and vulnerable, but not essential later,” says Agudo.
Agudo is pursuing this opportunity for early intervention from many angles, including efforts to better understand how the immune system detects abnormal cells, how SOX17 or one of its downstream effects could be targeted by drugs, and what turns SOX17 on in the first place.
“We really need to learn more about how cancers are able to hide from the immune system and grow,” says Agudo. “Hopefully by learning more about these early stages of cancer growth, we can develop therapies that can prevent that from happening or that can allow patients to respond to immunotherapy.”
