Bispecific antibody therapies are a type of immunotherapy for patients with B-cell non-Hodgkin lymphoma. These therapies provide a valuable new option for patients.
There are many standard therapies that are monoclonal antibodies. These therapies treat cancer by binding to a marker on cancer cells and rallying the immune system to destroy them.
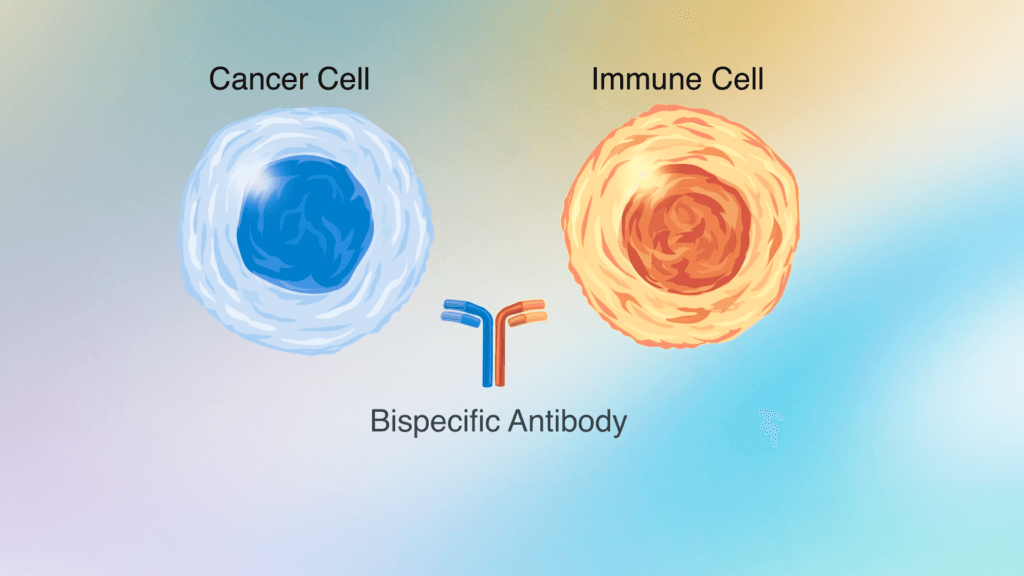
Bispecific antibodies are designed to recognize and bind to more than one cell marker. The bispecific antibodies approved for the treatment of B-cell non-Hodgkin lymphoma recognize CD20, a marker known to be on B cells, and CD3, a marker known to be on T cells, which are immune cells that can attack cancer cells.
By binding to both, the bispecific antibody pulls the T cells closer to the cancerous B cells, increasing the chances that the T cells will recognize and kill the cancer.
“Bispecific antibodies can be a powerful immune-based mechanism because of their dual binding properties,” says Jennifer Crombie, MD, a physician and clinical investigator in the Lymphoma Division of Dana-Farber.
Who are bispecific antibodies for?
Regulatory agencies around the world have so far approved three different bispecific antibodies for lymphoma: mosunetuzumab, epcoritamab, and glofitamab. All target CD3 and CD20.
These medicines are currently approved for use alone in patients who have received three lines of treatment and need a new therapeutic option. Mosunetuzumab is approved for follicular lymphoma and epcoritamab and glofitamab are approved for diffuse large B-cell lymphoma.
Some patients may choose to try bispecific antibody therapy after CAR T-cell therapy. Others may opt to try bispecific antibodies before trying or in lieu of CAR T-cell therapy. Recommendations will vary depending on the type of lymphoma and other individual factors.
How are bispecific antibodies delivered?
These therapies are administered via infusion or an injection under the skin, so they can be offered at a wide range of cancer care facilities. However, these immunotherapies can cause side effects that some care centers may still be learning how to manage.
To help centers handle side effects, an international team of experts with experience treating patients with bispecific antibody therapies joined together to create clinical guidelines. The guidelines will help prepare any cancer care center that offers them to manage their toxicities.
“The goal is to get these drugs to more patients safely,” says Crombie, co-lead author of the guidelines, which are published in Blood.
Crombie has run a clinical trial of a bispecific antibody therapy at Dana-Farber for the past several years. She and Philippe Armand, MD, PhD, chief of the Lymphoma Division at Dana-Farber, helped initiate this effort to create guidelines and provided expert guidance.
What side effects can bispecific antibody therapies cause?
In clinical trials of bispecific antibodies, investigators noted side effects that can become serious. However, these side effects didn’t occur in everyone and were manageable.
The side effects include:
- Cytokine release syndrome (CRS): This syndrome, also called a cytokine storm, is a whole-body inflammatory response that can cause a sudden onset of fever. Patients receiving bispecific antibody therapy most frequently experienced less serious grade 1 or grade 2 CRS and rarely experienced more serious higher grades.
- Neurologic toxicities: These include headaches and dizziness and were seen in small numbers of patients. Though rare, some patients experienced more serious neurotoxicity.
- Other side effects were also reported, including low blood counts and risks of infection.
Traditional medicines do not cause side effects such as CRS or neurologic toxicities that may require immediate intervention, so the management of them may be a new practice at some care centers.
These side effects do occur with CAR T-cell therapies, often more severely. Experts with experience with CAR T-cell therapies, such as oncologists at Dana-Farber and other major cancer centers, have applied what they have learned from managing CAR T-cell therapy toxicities to guide the management of bispecific antibody therapy toxicities.
How are side effects managed?
Side effects can be managed with medicines and interventions by trained care teams. They are managed as a team effort that includes the patient and caregivers, a care team of nurses and advanced practice providers, emergency room staff, oncologists, and pharmacists.
For patients and caregivers, instructions should include:
- Recommendations for monitoring the patient’s temperature, vital signs, and mental state, including confusion, difficulty speaking, and other symptoms.
- Details about who to call if symptoms occur and when to visit the emergency room.
- Information to provide to the care team about the patient’s treatment and condition so that responses are swift and appropriate.
For the care team, instructions should include:
- Recommendations for how to build and train a response team for managing side effects that includes the page operator, trained emergency room staff, nurses, advanced practice providers, and pharmacists with access to necessary medicines.
- Clinical protocols and available facilities for caring for patients with different medical needs.
- Resources, education, and materials for patients and caregivers.
Based on Crombie’s work, the hope is that facilities can educate providers who act as a team to field calls from patients, connect them to the right experts, and ensure care teams have the resources and medicines they need to respond.
“We hope that by having guidelines for managing these drugs, both academic and community cancer centers will feel more comfortable using them and that will open up access to them for patients more broadly,” says Crombie.
What new research is on the horizon related to bispecific antibodies?
Dana-Farber researchers are running clinical trials to determine whether bispecific antibodies could be effective in patients as the first line of therapy and if they could be effective in combination with other standard therapies.
- Crombie is leading trials for patients with diffuse large B-cell lymphoma.
- Reid Merryman, MD, a physician and clinical investigator in the Lymphoma Division, is leading trials for patients with follicular lymphoma.
- Christine Ryan, MD, a physician and clinical investigator in the Lymphoma Division, is leading a trial of a bispecific antibody for patients with Richter’s syndrome, which occurs when CLL transforms to an aggressive lymphoma.
Learn more about bispecific antibody therapies in lymphoma clinical trials at Dana-Farber.
